Abstract
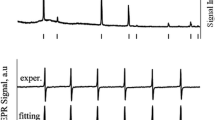
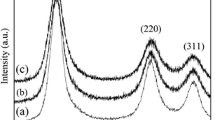
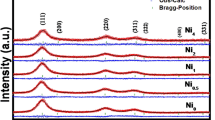
The structural changes of cubic ZnS (cZnS) nanocrystals (NCs) doped with 0.2 at.% Mn2+ pulse annealed in vacuum and in air, up to 500 °C, were investigated by multifrequency electron paramagnetic resonance (EPR), X-ray diffraction (XRD), and transmission electron microscopy (TEM). The samples, prepared by a surfactant (Tween20)-assisted liquid–liquid reaction at pH = 6, consist of NCs with a tight size distribution around 3 nm and high crystallinity self-assembled into a stable mesoporous structure. The EPR spectra of the as prepared samples contain only the characteristic lines of the substitutional Mn2+(I) centers. No spectra from Mn2+ ions localized in (hydro)oxidized regions of the NCs surface were observed. The absence of such a surface layer could explain the stability of the cubic (sphalerite) structure observed by XRD and TEM in the investigated cZnS:Mn NCs annealed in vacuum up to 500 °C. The observation of the cubic-hexagonal transformation for the same NCs annealed in air supports the role of such layer in promoting this structural transformation. The narrowing of the EPR spectral lines above 200 °C with the increase in the average size of the cZnS:Mn crystallites was observed. The effect was more pronounced for the sample annealed in air. EPR also revealed the formation of minute amounts of substitutional Mn2+-type centers in a hexagonal ZnO structure at T ~ 300 °C, corresponding to the early stages of the thermally induced oxidation of the cZnS:Mn NCs.







Similar content being viewed by others
References
Abragam A, Bleaney B (1970) Electron paramagnetic resonance of transition ions. Clarendon Press, Oxford
Beaulac R, Ochsenbein ST, Gamelin DR (2010) Colloidal transition metal-doped quantum dots. In: Klimov VI (ed) Nanocrystal quantum dots, 2nd edn. CRC Press, Boca Raton, pp 398–453
Diaconu M, Schmidt H, Poeppl A, Boettcher R, Hoentsch J, Klunker A, Spemann D, Hochmuth H, Lorentz M, Grundmann M (2005) Electron paramagnetic resonance of Zn1−x Mn x O thin films and single crystals. Phys Rev B 72:085214(6)
Dinsmore AD, Hsu DS, Qadri SB, Cross JO, Kennedy TA, Gray HF, Ratna BR (2000) Structure and luminescence of annealed nanoparticles of ZnS:Mn. J Appl Phys 88:4985–4993
Keller SP, Gelles IL, Smith WV (1958) Paramagnetic resonance absorption in Mn-activated hexagonal Zns. Phys Rev 110:850–855
Murakoshi K, Hosokawa H, Tanaka N, Saito M, Wada Y, Sakata T, Mori H, Yanagida S (1998) Phase transition of ZnS nanocrystallites induced by surface modification at ambient temperature and pressure confirmed by electron diffraction. Chem Commun 321–322
Nistor SV, Stefan M (2009) In-depth investigation of EPR spectra of Mn2+ ions in ZnS single crystals with pure cubic structure. J Phys: Condens Matter 21:145408(7)
Nistor LC, Mateescu CD, Birjega R, Nistor SV (2008) Synthesis and characterization of mesoporous ZnS with narrow size distribution of small pores. Appl Phys A: Mater Sci Process 92:295–301
Nistor SV, Nistor LC, Stefan M, Mateescu CD, Birjega R, Solovieva N, Nikl M (2009) Synthesis and characterization of Mn2+ doped ZnS nanocrystals self-assembled in a tight mesoporous structure. Superlattices Microstruct 46:306–311
Nistor SV, Nistor LC, Stefan M, Ghica D, Mateescu CD, Birjega R (2010a) Improving the cubic ZnS nanocrystals quality by self-assembling into a mesoporous structure. Rom Rep Phys 62:319–328
Nistor SV, Stefan M, Nistor LC, Ghica D, Mateescu CD, Barascu JN (2010b) Lattice defect assisted incorporation of Mn2+ ions in cubic II-VI semiconductor quantum dots. IOP Conf Series: Mat Sci Eng 15:012024(7)
Nistor SV, Stefan M, Nistor LC, Goovaerts E, Van Tendeloo G (2010c) Incorporation and localization of substitutional Mn2+ ions in cubic ZnS quantum dots. Phys Rev B 81:035336(6) and references cited therein
Nistor SV, Stefan M, Nistor LC, Mateescu CD, Birjega R (2010d) Localization of Mn2+ ions in mesoporous ZnS. J Nanosci Nanotechnol 10:6200–6205
Qadri SB, Skelton EF, Hsu D, Dinsmore AD, Yang J, Gray HF, Ratna BR (1999) Size-induced transition-temperature reduction in nanoparticles of ZnS. Phys Rev B 60:9191–9193
Simanek E, Muller KA (1970) Covalency and hyperfine structure constant A of iron group impurities in crystals. J Phys Chem Sol 31:1027–1040
Stefan M, Nistor SV, Barascu JN (2011a) Accurate determination of the spin Hamiltonian parameters for Mn2+ ions in cubic ZnS nanocrystals by multifrequency EPR spectra analysis. J Magn Res doi:10.1016/j.jmr.2011.03.004
Stefan M, Nistor SV, Ghica D, Mateescu CD, Nikl M, Kucerkova R (2011b) Substitutional and surface Mn2+ centers in small cubic ZnS:Mn nanocrystals. A correlated EPR and photoluminescence study. Phys Rev B 83:045301(11)
Stoll S, Schweiger A (2006) EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J Magn Res 178:42–55
Stoneham AM (1969) Shapes of inhomogeneously broadened resonance lines in solids. Rev Mod Phys 41:82–108
Trueba A, Garcia-Lastra JM, Barriuso MT, Arambaru JA, Moreno M (2008) Influence of internal electric field on bonding and properties of impurities in insulators: Mn2+ in LiBaF3 and normal perovskites. Phys Rev B 78:075108(11)
Vogel W, Borse PH, Deshmukh N, Kulkarni SK (2000) Structure and stability of monodisperse 1.4-nm ZnS particles stabilized by mercaptoethanol. Langmuir 16:2032–2037
Yosida T (1980) The ENDOR investigations of LiBaF3: Mn2+ having the inverse perovskite structure. J Phys Soc Jpn 49:127–135
Zhang H, Banfield JF (2009) Identification and growth mechanism of ZnS nanoparticles with mixed cubic and hexagonal stacking. J Phys Chem C 113:9681–9687
Zhang H, Gilbert B, Huang F, Banfield JF (2003a) Water-driven structure transformation in nanoparticles at room temperature. Nature 424:1025–1029
Zhang H, Huang F, Gilbert B, Banfield JF (2003b) Molecular dynamics simulations, thermodynamic analysis, and experimental study of phase stability of zinc sulfide nanoparticles. J Phys Chem B 107:13051–13060
Zhou H, Hofmann DM, Hofstaetter A, Meyer BK (2003) Magnetic resonance investigation of Mn2+ in ZnO nanocrystals. J Appl Phys 94:1965–1968
Acknowledgments
This work was supported by CNCSIS–UEFISCSU, Project Number PNII-IDEI-523/2008. The authors are grateful to D. Zernescu for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghica, D., Nistor, S.V., Nistor, L.C. et al. Structural phase transformations in annealed cubic ZnS nanocrystals. J Nanopart Res 13, 4325–4335 (2011). https://doi.org/10.1007/s11051-011-0379-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-011-0379-y