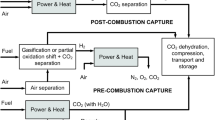
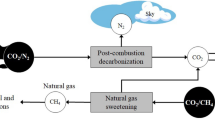
Abstract
Membrane gas separation technology has been rapidly growing for industrial applications such as air separation, carbon dioxide (CO2) separation from natural gas production, hydrogen separation, etc. Needs for CO2 separation are increasing as carbon capture technology has been recognized as an essential part when combating the global warming issue. Membrane gas separation technology deals with mass transport phenomena through the membrane engineered on a sub-nanoscale controlling transport properties of small gas molecules such as CO2, N2, O2, H2, etc. In this review, we will report on the recent developments in capture technologies utilizing various membranes including nano-engineered thermally rearranged (TR) polymers. TR polymer membranes show high gas permeability as well as good separation properties, especially in CO2 separation processes such as from post-combustion flue gas and natural gas sweetening.





Similar content being viewed by others
References
Allcock HR, Lampe FW, Mark JE (2003) Contemporary polymer science. Pearson Education Inc., Upper Saddle River
Ammala A, Hill AJ, Meakin P, Pas SJ, Turney TW (2002) Degradation studies of polyolefins incorporating transparent nanoparticulate zinc oxide UV stabilizers. J Nanopart Res 4:167–174
Anderson CJ, Pas SJ, Arora G, Kentish SE, Hill AJ, Sandler SI, Stevens GW (2008) Effect of pyrolysis temperature and operating temperature on the performance of nanoporous carbon membranes. J Membr Sci 322:19–27
Bae YS, Snurr RQ (2011) Development and evaluation of porous materials for carbon dioxide separation and capture. Angew Chem Int Ed 50:11586–11596
Baker RW (2002) Future directions of membrane gas separation technology. Ind Eng Chem Res 41:1393–1411
Banerjee R, Phan A, Wang B, Knobler C, Furukawa H, O’Keeffe M, Yaghi OM (2008) High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 319:939–943
Bernardo P, Drioli E, Golemme G (2009) Membrane gas separation: a review/state of the art. Ind Eng Chem Res 48:4638–4663
Bos A, Pünt I, Strathmann H, Wessling M (2001) Suppression of gas separation membrane plasticization by homogeneous polymer blending. AIChE J 47:1088–1093
Brunetti A, Scura F, Barbieri G, Drioli E (2010) Membrane technologies for CO2 separation. J Membr Sci 359:115–125
Budd PM, McKeown NB, Fritsch D (2006) Polymers of intrinsic microporosity (PIMs): high free volume polymers for membrane applications. Macromol Symp 245–246:403–405
Calle M, Lee YM (2011) Thermally rearranged (TR) poly(ether-benzoxazole) membranes for gas separation. Macromolecules 44:1156–1165
Choi JI, Jung CH, Han SH, Park HB, Lee YM (2010) Thermally rearranged (TR) poly(benzoxazole-co-pyrrolone) membranes tuned for high gas permeability and selectivity. J Membr Sci 349:358–368
Chu S (2009) Carbon capture and sequestration. Science 325:1599
Clausi DT, Koros WJ (2000) Formation of defect-free polyimide hollow fiber membranes for gas separations. J Membr Sci 167:79–89
Cui L, Qiu W, Paul DR, Koros WJ (2011) Physical aging of 6FDA-based polyimide membranes monitored by gas permeability. Polymer 52:3374–3380
Das M, Koros WJ (2010) Performance of 6FDA–6FpDA polyimide for propylene/propane separations. J Membr Sci 365:399–408
Dasgupta B, Sen SK, Banerjee S (2010) Aminoethylaminopropylisobutyl POSS—polyimide nanocomposite membranes and their gas transport properties. Mater Sci Eng B 168:30–35
Du N, Park HB, Robertson GP, Dal-Cin MM, Visser T, Scoles L, Guiver MD (2011) Polymer nanosieve membranes for CO2-capture applications. Nat Mater 10:372–375
Freeman B, Yampolskii Y, Pinnau I (2006) Materials science of membranes for gas and vapor separation. Wiley, Chichester
Gibbins J, Chalmers H (2008) Carbon capture and storage. Energy Policy 36:4317–4322
Gramm F, Baerlocher C, McCusker LB, Warrender SJ, Wright PA, Han B, Hong SB, Liu Z, Ohsuna T, Terasaki O (2006) Complex zeolite structure solved by combining powder diffraction and electron microscopy. Nature 444:79–81
Han SH, Lee JE, Lee KJ, Park HB, Lee YM (2010a) Highly gas permeable and microporous polybenzimidazole membrane by thermal rearrangement. J Membr Sci 357:143–151
Han SH, Misdan N, Kim S, Doherty CM, Hill AJ, Lee YM (2010b) Thermally rearranged (TR) polybenzoxazole: effects of diverse imidization routes on physical properties and gas transport behaviors. Macromolecules 43:7657–7667
Herzog HJ (2001) Peer reviewed: what future for carbon capture and sequestration? Environ Sci Technol 35:148A–153A
Idem R, Wilson M, Tontiwachwuthikul P, Chakma A, Veawab A, Aroonwilas A, Gelowitz D (2005) Pilot plant studies of the CO2 capture performance of aqueous MEA and mixed MEA/MDEA solvents at the University of Regina CO2 capture technology development plant and the boundary dam CO2 capture demonstration plant. Ind Eng Chem Res 45:2414–2420
Imai Y, Taoka I, Uno K, Iwakura Y (1965) Polybenzoxazoles and polybenzothiazoles. Die Makromol Chem 83:167–178
Jung CH, Lee JE, Han SH, Park HB, Lee YM (2010) Highly permeable and selective poly(benzoxazole-co-imide) membranes for gas separation. J Membr Sci 350:301–309
Kazama S (2004) In: CO2 separation with molecular gate membrane. GCEP Energy Workshops, Stanford, Palo Alto, CA
Kim S, Han SH, Lee YM (2012) Thermally rearranged (TR) polybenzoxazole hollow fiber membranes for CO2 capture. J Membr Sci 403–404:169–178
Koros WJ, Fleming GK (1993) Membrane-based gas separation. J Membr Sci 83:1–80
Kosuri MR, Koros WJ (2008) Defect-free asymmetric hollow fiber membranes from Torlon®, a polyamide-imide polymer, for high-pressure CO2 separations. J Membr Sci 320:65–72
Li Y, Chung T-S (2010) Molecular-level mixed matrix membranes comprising Pebax® and POSS for hydrogen purification via preferential CO2 removal. Int J Hydrogen Energy 35:10560–10568
Lin K-Y, Wang D-M, Lai J-Y (2002) Nonsolvent-Induced gelation and its effect on membrane morphology. Macromolecules 35:6697–6706
Marchant G, White A (2011) An international nanoscience advisory board to improve and harmonize nanotechnology oversight. J Nanopart Res 13:1489–1498
McKeown NB, Budd PM (2010) Exploitation of intrinsic microporosity in polymer-based materials. Macromolecules 43:5163–5176
McKeown NB, Gahnem B, Msayib KJ, Budd PM, Tattershall CE, Mahmood K, Tan S, Book D, Langmi HW, Walton A (2006) Towards polymer-based hydrogen storage materials: engineering ultramicroporous cavities within polymers of intrinsic microporosity. Angew Chem Int Ed 45:1804–1807
Merkel TC, He Z, Pinnau I, Freeman BD, Meakin P, Hill AJ (2003) Effect of nanoparticles on gas sorption and transport in poly(1-trimethylsilyl-1-propyne). Macromolecules 36:6844–6855
Merkel TC, Lin H, Wei X, Baker R (2010) Power plant post-combustion carbon dioxide capture: an opportunity for membranes. J Membr Sci 359:126–139
Mulder M (1996) Basic principles of membrane technology. Kluwer Academic Publishers, Dordrecht
Park HB, Jung CH, Lee YM, Hill AJ, Pas SJ, Mudie ST, Van Wagner E, Freeman BD, Cookson DJ (2007) Polymers with cavities tuned for fast selective transport of small molecules and ions. Science 318:254–258
Park HB, Han SH, Jung CH, Lee YM, Hill AJ (2010) Thermally rearranged (TR) polymer membranes for CO2 separation. J Membr Sci 359:11–24
Pham TCT, Kim HS, Yoon KB (2011) Growth of uniformly oriented silica MFI and BEA zeolite films on substrates. Science 334:1533–1538
Pires JCM, Martins FG, Alvim-Ferraz MCM, Simões M (2011) Recent developments on carbon capture and storage: an overview. Chem Eng Res Des 89:1446–1460
Powell CE, Qiao GG (2006) Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J Membr Sci 279:1–49
Rao AB, Rubin ES (2002) A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ Sci Technol 36:4467–4475
Robeson LM (2008) The upper bound revisited. J Membr Sci 320:390–400
Rochelle GT (2009) Amine scrubbing for CO2 capture. Science 325:1652–1654
Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O’Keeffe M, Yaghi OM (2003) Hydrogen storage in microporous metal-organic frameworks. Science 300:1127–1129
Rowsell JLC, Millward AR, Park KS, Yaghi OM (2004) Hydrogen sorption in functionalized metal–organic frameworks. J Am Chem Soc 126:5666–5667
Rowsell JLC, Yaghi OM, Chen B, Ockwig NW, Millward AR, Contreras DS (2005) Cover picture: strategies for hydrogen storage in metal–organic frameworks/high H2 adsorption in a microporous metal–organic framework with open metal sites. Angew Chem Int Ed 44:4647
Shin J, Camblor MA, Woo HC, Miller SR, Wright PA, Hong SB (2009) PST-1: a synthetic small-pore zeolite that selectively adsorbs H2. Angew Chem Int Ed 121:6775–6777
Staiger CL, Pas SJ, Hill AJ, Cornelius CJ (2008) Gas separation, free volume distribution, and physical aging of a highly microporous spirobisindane polymer. Chem Mater 20:2606–2608
Terry FW (2007) Combustion processes for carbon capture. Proc Combust Inst 31:31–47
Thomas S, Pinnau I, Du N, Guiver MD (2009) Pure- and mixed-gas permeation properties of a microporous spirobisindane-based ladder polymer (PIM-1). J Membr Sci 333:125–131
Tin PS, Chung T-S, Liu Y, Wang R (2004) Separation of CO2/CH4 through carbon molecular sieve membranes derived from P84 polyimide. Carbon 42:3123–3131
Tullos G, Mathias L (1999) Unexpected thermal conversion of hydroxy-containing polyimides to polybenzoxazoles. Polymer 40:3463–3468
Tullos GL, Powers JM, Jeskey SJ, Mathias LJ (1999) Thermal conversion of hydroxy-containing imides to benzoxazoles: polymer and model compound study. Macromolecules 32:3598–3612
Venna SR, Carreon MA (2009) Highly permeable zeolite imidazolate framework-8 membranes for CO2/CH4 separation. J Am Chem Soc 132:76–78
Wang L, Cao Y, Zhou M, Liu Q, Ding X, Yuan Q (2008) Gas transport properties of 6FDA-TMPDA/MOCA copolyimides. Eur Polym J 44:225–232
Zhao L, Riensche E, Menzer R, Blum L, Stolten D (2008) A parametric study of CO2/N2 gas separation membrane processes for post-combustion capture. J Membr Sci 325:284–294
Acknowledgments
This work was financially supported by Korea CCS R&D Center (KCRC), funded by the Ministry of Education, Science and Technology in Korea. YML appreciates support from WCU (World Class University) program, National Research Foundation (NRF) of the Korean Ministry of Science and Technology (No. R31-2008-000-10092-0), which we gratefully acknowledge.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue Editors: Mamadou Diallo, Neil Fromer, Myung S. Jhon
This article is part of the Topical Collection on Nanotechnology for Sustainable Development
Rights and permissions
About this article
Cite this article
Kim, S., Lee, Y.M. Thermally rearranged (TR) polymer membranes with nanoengineered cavities tuned for CO2 separation. J Nanopart Res 14, 949 (2012). https://doi.org/10.1007/s11051-012-0949-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0949-7