Abstract
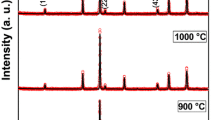
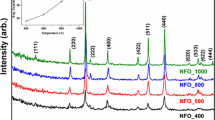
This study demonstrated the possibility of using microwave heating as a fast and cheap method for synthesizing superparamagnetic nanoparticles. In this sense, NiFe2O4 samples were subjected to microwave heating at various temperatures to determine the lowest temperature at which the crystalline phase of the nanoparticles occurs. X-Ray powder diffraction, 57Fe Mössbauer spectroscopy, and transmission electron microscopy of the samples were performed to confirm the formed nanoparticles. It was observed a cubic structure of inverse spinel type with good crystallinity. The magnetic properties of the samples were studied using a vibrating sample magnetometer and was found to zero values to remanent magnetization and coercivity field. This behavior suggests superparamagnetic features for all samples. The crystallite size (9, 10, and 12 nm) and saturation magnetization (31–45 emu/g) were used as a function of the increase of the temperature treatment time. Blocking temperature was found by tracing remanent magnetization versus temperature.







Similar content being viewed by others
References
Amer MA, Meaz TM et al (2005) Mössbauer, infrared and X-ray studies of Ni0.5Zn0.5CrxFe2-xO4 ferrites. Egyp J Solids 28:3
Barreto A, Santiago V et al (2011) Magnetic nanoparticles for a new drug delivery system to control quercetin releasing for cancer chemotherapy. J Nanopart Res 13(12):6545–6553
Bennett CO, Myers JE (1982) Momentum, heat, and mass transfer. McGraw-Hill, New York
Bleicher L, Sasaki JM et al (2000) Development of a graphical interface for the Rietveld refinement program DBWS. J Appl Crystallogr 33(4):1189
Braga TP, Vasconcelos IF et al (2010) Magnetic composites based on hybrid spheres of aluminum oxide and superparamagnetic nanoparticles of iron oxides. J Magn Magn Mater 322(6):633–637
Cullity BD, Graham CD (2011) Introduction to magnetic materials. Wiley, New York
Dickson DPE, Berry FJ (1986) Mössbauer spectroscopy. Cambridge University Press, Cambridge
Freire R, Ribeiro T et al (2013) MZnFe2O4 (M=Ni, Mn) cubic superparamagnetic nanoparticles obtained by hydrothermal synthesis. J Nanopart Res 15(5):1–12
Friedlander SK (2000) Smoke, dust, and haze. Oxford University Press, New York
Gibb TC (1994) Encyclopedia of Inorganic Chemistry. Wiley, Chidrester
Karakas ZK, Boncukcuoglu R et al (2013) The Investigation of the Removal of the Arsenic from Wastewaters by Using NiFe2O4 Nanoparticles Produced with Microwave Assisted Combustion Method. J Selcuk Univ Nat Appl Sci 2013:332–338
Klabunde KJ, Richards R (2001) Nanoscale materials in chemistry. Wiley, New York
Koziej D, Floryan C et al (2013) Microwave dielectric heating of non-aqueous droplets in a microfluidic device for nanoparticle synthesis. Nanoscale 5(12):5468–5475
Latham AH, Williams ME (2008) Controlling Transport and Chemical Functionality of Magnetic Nanoparticles. Acc Chem Res 41(3):411–420
Markov IV (1995) Crystal growth for beginners: fundamentals of nucleation, crystal growth, and epitaxy. World Scientific, Singapore
Patterson A (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56(10):978
Petcharoen K, Sirivat A (2012) Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater Sci Eng, B 177(5):421–427
Rietveld HM (1967) Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallography 22:151–152
Thomas JJ, Shinde AB, Krishna PSR, Kalarikkal N (2013) Cation distribution and micro level magnetic alignments in the nanosized nickel zinc ferrite. J Alloy Compd 546:77–83. doi:10.1016/j.jallcom.2012.08.011
Tong J, Cai X et al (2013) Efficient magnetic CoFe2O4 nanocrystal catalyst for aerobic oxidation of cyclohexane prepared by sol–gel auto-combustion method: effects of catalyst preparation parameters. J Sol-Gel Sci Technol 66(3):452–459
Wang L, Li FS (2001) Mossbauer study of nanocrystalline Ni-Zn ferrite. J Magn Magn Mater 223(3):233–237
Weidler P, Luster J et al (1998) The Rietveld method applied to the quantitative mineralogical and chemical analysis of a ferralitic soil. Eur J Soil Sci 49(1):95–105
Williamson G, Hall W (1953) X-ray line broadening from filed aluminium and wolfram. Acta Metall 1(1):22–31
Yamaura M, Camilo R et al (2004) Preparation and characterization of (3-aminopropyl) triethoxysilane-coated magnetite nanoparticles. J Magn Magn Mater 279(2):210–217
Zhang Z-G, YAO G-C et al (2010) Synthesis of NiFe2O4 spinel nanopowder via low-temperature solid-state reactions. J Northeastern Univ (Nat Sci) 31(6):868–872
Zhang W, Jia S et al (2012) Studies of the magnetic field intensity on the synthesis of chitosan-coated magnetite nanocomposites by co-precipitation method. Mater Sci Eng, C 32(2):381–384
Zhang W, Jia S-Y et al (2013) Effects of alkaline precipitating agents on synthesis of magnetite nanomaterials by hydrothermal d-glucose method. J Nanopart Res 15(6):1–6
Acknowledgments
This work was partly sponsored by CAPES, CNPq, and FUNCAP (Brazilian agencies). The support from Fondecyt 1140195, Millennium Science Nucleus, Basic and Applied Magnetism Grant N°P10-061-F, and CONICYT BASAL CEDENNA FB0807 is gratefully acknowledged (Chilean agencies). The authors acknowledge the Laboratório Nacional de Nanotecnologia (LNNano/CNPEM) for providing the equipment and technical support for the experiments involving transmission electron microscopy (TEM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galvão, W.S., Freire, R.M., Ribeiro, T.S. et al. Cubic superparamagnetic nanoparticles of NiFe2O4 via fast microwave heating. J Nanopart Res 16, 2803 (2014). https://doi.org/10.1007/s11051-014-2803-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2803-6