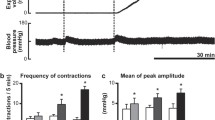
Immunohistochemical and neurophysiological experiments on rats were performed to study the responses of solitary tract nucleus neurons to nociceptive stimulation of the large intestine and to identify the role of nitric oxide in these processes. Nociceptive distension of the colorectal part of the intestine was found to induce significant increases in the level of expression of c-fos proteins, mainly in the medial, commissural, parvocellular, and dorsomedial subnuclei of the solitary tract. Neurophysiological experiments showed that non-painful colorectal distension induced mild excitatory responses in cells in these structures, these being exclusively of the phasic type. Painful stimulation of the intestine was accompanied by significant increases in the responses of these neurons and activation of neurons with tonic responses. Blockade of neuronal NO synthase led to significant decreases in neuron responses to nociceptive distension of the intestine and the number of cells with tonic responses. It is suggested that neurons with NO-dependent tonic responses may be directly associated with the transmission of pain information from the large intestine.
Similar content being viewed by others
References
O. A. Lyubashina, S. S. Panteleev, and A. A. Nozdrachev, Amygdalofugal Modulation of the Autonomic Centers of the Brain [in Russian], Nauka, St. Petersburg (2009).
S. S. Panteleev, V. A. Bagaev, and A. D. Nozdrachev, Cortical Modulation of Visceral Reflexes [in Russian], St. Petersburg State University Press, St. Petersburg (2004).
L. V. Filippova and A. D. Nozdrachev, “Current concepts in the mechanisms encoding visceral pain stimuli,” Fiziol. Cheloveka, 36, No. 1, 125–137 (2010).
E. D. Al-Chaer and R. J. Traub, “Biological basis of visceral pain: recent developments,” Pain, 96, No. 3, 221–225 (2002).
E. D. Al-Chaer and W. D. Willis, “Neuroanatomy of visceral pain: pathways and processes,” in: Chronic Abdominal and Visceral Pain. Theory and Practice, Section II: The Neurobiology and Psychobiology of Chronic Visceral Pain, P. L. Pasricha, W. D. Willis, and G. F. Gebhart (eds.) Informa Healthcare, New York (2007), pp. 33–44.
S. M. Altschuler, J. Escardo, R. B. Lynn, and R. R. Miselis, “The central organization of the vagus nerve innervating the colon of the rat,” Gastroenterology, 104, No. 2, 502–509 (1993).
K. J. Berkley, C. H. Hubscher, and P. D. Wall, “Neuronal responses to stimulation of the cervix, uterus, colon, and skin in the rat spinal cord,” J. Neurophysiol., 69, No. 2, 545–556 (1993).
H. R. Berthoud, and W. L. Neuhuber, “Functional and chemical anatomy of the afferent vagal system,” Auton. Neurosci., 85, No. 1–3, 1–17 (2000).
K. Bielefeldt, “Neurochemical and molecular basis of peripheral sensitization,” in: Chronic Abdominal and Visceral Pain. Theory and Practice, Section II: The Neurobiology and Psychobiology of Chronic Visceral Pain, P. L. Pasricha, W. D. Willis, and G. F. Gebhart (eds.) Informa Healthcare, New York (2007), pp. 67–84.
A. C. Dias, E. Colombari, and S. W. Miflin, “Effect of nitric oxide on excitatory amino acid-evoked discharge of neurons in NTS,” Am. J. Physiol. Heart Circ. Physiol., 284, No. 1, H234–H240 (2003).
F. O. Gamboa-Esteves, P. N. McWilliam, and T. F. Batten, “Substance P (NK1) and somatostatin (sst2A) receptor immunoreactivity in NTS-projecting rat dorsal horn neurones activated by nociceptive afferent input,” J. Chem. Neuroanat., 27, No. 4, 251–266 (2004).
S. M. Hsu, L. Raine, and H. Fanger, “Use of avidin-biotin complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedure,” J. Histochem. Cytochem., 29, No. 4, 577–580 (1981).
M. Kalia and D. Richter, “Rapidly adapting pulmonary receptor afferents: I. Arborization in the nucleus of the tractus solitarius,” J. Comp. Neurol., 274, No. 4, 560–573 (1988).
C. H. Knowles and Q. Aziz, “Basic and clinical aspects of gastrointestinal pain,” Pain, 141, No. 3, 191–209 (2009).
L.-H. Lin, “Glutamatergic neurons say NO in the nucleus tractus solitarii,” J. Chem. Neuroanat., 38, No. 3, 154–165 (2009).
V. Martinez, L. Wang, and Y. Taché, “Proximal colon distension induces Fos expression in the brain and inhibits gastric emptying through capsaicin-sensitive pathways in conscious rats,” Brain Res., 1086, No. 1, 168–180 (2006).
M. Isamu, Y. Hirooka, K. Hironaga, et al., “Glutamate release via NO production evoked by NMDA in the NTS enhances hypotension and bradycardia in vivo,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 280, No. 5, R1285-R1291 (2001).
A. Miclescu and T. Gordh, “Nitric oxide and pain: Something old, something new,” Acta Anesthesiol. Scand., 53, No. 9, 1107–1120 (2009).
H. Mönnikes, J. Rüter, M. König, et al., “Differential induction of c-fos expression in brain nuclei by noxious and non-noxious colonic distension: role of afferent C-fibers and 5-HT3 receptors,” Brain Res., 966, No. 2, 253–264 (2003).
T. J. Ness, “Distinctive clinical and biological characteristics of visceral pain,” in: Chronic Abdominal and Visceral Pain. Theory and Practice, Section II: The Neurobiology and Psychobiology of Chronic Visceral Pain, P. L. Pasricha, W. D. Willis, and G. F. Gebhart (eds.) Informa Healthcare, New York (2007), pp. 1–10.
T. J. Ness and G. F. Gebhart, “Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat,” J. Neurophysiol., 57, No. 6, 1867–1892 (1987).
T. J. Ness and G. F. Gebhart, “Colorectal distension as a noxious visceral stimulus: Physiologic and pharmacologic characterization of pseudoaffective reflexes in the rat,” Brain Res., 450, No. 1–2, 153–169 (1988).
T. J. Ness and G. F. Gebhart, “Characterization of neurons responsive to noxious colorectal distension in the T13-L2 spinal cord of the rat,” J. Neurophysiol., 60, No. 4, 1419–1438 (1988).
T. J. Ness and G. F. Gebhart, “Visceral pain: a review of experimental studies,” Pain, 41, No. 2, 167–234 (1990).
A. Ohta, H. Takagi, T. Matsui, et al., “Localization of nitric oxide synthase immunoreactive neurons in the solitary nucleus and ventrolateral medulla oblongata of the rat: their relation to catecholaminergic neurons,” Neurosci. Lett., 158, No. 1, 33–35 (1993).
G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, New York (1998).
H. Prast and A. Philippu, “Nitric oxide as modulator of neuronal function,” Progr. Neurobiol., 64, No. 1, 51–68 (2001).
J. E. Torres, N. R. Kreisman, and D. Gozal, “Nitric oxide modulates in vitro intrinsic optical signal and neural activity in the nucleus tractus solitarius of the rat,” Neurosci. Lett., 232, No. 3, 175–178 (1997).
R. J. Traub, “Spinal mechanisms of visceral pain and sensitization,” in: Chronic Abdominal and Visceral Pain. Theory and Practice, Section II: The Neurobiology and Psychobiology of Chronic Visceral Pain, P. L. Pasricha, W. D. Willis, and G. F. Gebhart (eds.) Informa Healthcare, New York (2007), pp. 85–106.
R. J. Traub, “Evidence for thoracolumbar spinal cord processing of inflammatory, but not acute colonic pain,” Neuroreport, 11, No. 10, 2113–2116 (2000).
R. J. Traub, T. Herdegen, and G. F. Gebhart, “Differential expression of c-fos and c-jun in two regions of the rat spinal cord following noxious colorectal distention,” Neurosci. Lett., 160, No. 2, 121–125 (1993).
R. J. Traub, J. N. Sengupta, and G. F. Gebhart, “Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distension in the rat,” Neuroscience, 74, No. 3, 873–884 (1996).
G. Wang, B. Tang, and R. J. Traub, “Differential processing of noxious colonic input by thoracolumbar and lumbosacral dorsal horn neurons in the rat,” J. Neurophysiol., 94, No. 6, 3788–3794 (2005).
S. Wang, J. F. Paton, and S. Kasparov, “Differential sensitivity of excitatory and inhibitory synaptic transmission to modulation by nitric oxide in rat nucleus tractus solitarii,” Exp. Physiol., 92, No. 2, 371–382 (2007).
C. C. Wang, W. D. Willis, and K. N. Westlund, “Ascending projections from the area around the spinal cord central canal: A Phaseolus vulgaris leucoagglutinin study in rats,” J. Comp. Neurol., 415, No. 3, 341–367 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 97, No. 12, pp. 1336–1345, December, 2011.
Rights and permissions
About this article
Cite this article
Panteleev, S.S., Martseva, A.A. & Lyubashina, O.A. Responses of Solitary Tract Nucleus Neurons to Nociceptive Stimuli of the Large Intestine in Rats. Neurosci Behav Physi 43, 775–781 (2013). https://doi.org/10.1007/s11055-013-9808-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-013-9808-y