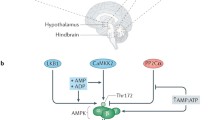
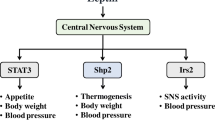
Metformin (MF) is the most widely used drug for the treatment of type 2 diabetes mellitus and metabolic syndrome (MS). In the peripheral tissues, MF inhibits mitochondrial respiratory chain complex I and increases the activity of AMP-activated protein kinase (AMPK), leading to suppression of gluconeogenesis in the liver, increased insulin sensitivity, increased glucose utilization, and normalization of lipid metabolism. Recent years have seen the appearance of experimental and clinical evidence showing that the CNS is also a major target of MF, such that the action of MF on the functional state of neurons can be mediated both via AMPK-dependent and via AMPK-independent signal cascades. In contrast to the periphery, MF does not activate but suppresses AMPK activity in hypothalamic neurons, this influencing the ratio of anorexigenic (melanocortin peptides) and orexigenic (neuropeptide Y) factors and altering feeding behavior. A significant contribution to this effect is made by MF-induced activation of the leptin-dependent STAT3 signal cascade. As the leptin and melanocortin systems targeted by MF interact closely with the insulin and monoamine systems, MF, acting through them, affects the entire integrative signal system of the brain, on which the functioning of the nervous, endocrine, cardiovascular, and other body systems depend. This review analyzes and systematizes data on the molecular mechanisms and targets of the action of MF in brain neurons, and the effects of this drug, which are mediated or may be mediated by the interactions of MF with the CNS.
Similar content being viewed by others
References
K. V. Derkach, L. A. Kuznetsova, T. S. Sharova, P. A. Ignat’eva, V. M. Bondareva, and A. O. Shpakov, “Effects of prolonged metformin treatment on the activity of the adenylate cyclase system and NO synthase in the brain and myocardium of rats with obesity,” Tsitologiya, 57, No. 5, 360–369 (2015).
K. V. Derkach, I. B. Sukhov, L. A. Kuznetsova, D. M. Buzanakov, and A. O. Shpakov, “Functional activity of hypothalamic signal systems during metformin treatment of rats with neonatal diabetes mellitus,” Dokl. Akad. Nauk., 467, No. 2, 222–225 (2016).
A. O. Shpakov, “The leptin signal system of the brain and its functional state in conditions of metabolic syndrome and type 2 diabetes mellitus,” Zh. Evolyuts. Biokhim. Fiziol., 52, No. 3, 161–176 (2016).
A. O. Shpakov and K. V. Derkach, “The melanocortin signal system of the hypothalamus and its functional state in conditions of type 2 diabetes mellitus and metabolic syndrome,” Ros. Fiziol. Zh. im. I. M. Sechenova, 102, No. 1, 18–40 (2016).
H. An and L. He, “Current understanding of metformin effect on the control of hyperglycemia in diabetes,” J. Endocrinol., 228, No. 3, R97–R106 (2016).
G. Aubert, V. Mansuy, M. J. Voirol, L. Pellerin, and F. P. Pralong, “The anorexigenic effects of metformin involve increases in hypothalamic leptin receptor expression,” Metabolism, 60, No. 3, 327–334 (2011).
M. P. Baruah, S. Kalra, and S. Ranabir, “Metformin; A character actor in the leptin story!,” Ind. J. Endocrinol. Metab., 16 (Supplement 3), S532–S533 (2012).
J. A. Bayliss, M. B. Lemus, V. V. Santos, M. Deo, J. S. Davies, B. E. Kemp, J. D. Elsworth, and Z. B. Andrews, “Metformin prevents nigrostriatal dopamine degeneration independent of AMPK activation in dopamine neurons,” PLoS One, 11, No. 7, e0159381 (2016).
M. S. Beeri, J. Schmeidler, J. M. Silverman, S. Gandy, M. Wysocki, C. M. Hannigan, D. P. Purohit, G. Lesser, H. T. Grossman, and V. Haroutunian, “Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology,” Neurology, 71, 750–757 (2008).
R. Carvajal, C. Rosas, K. Kohan, F. Gabler, D. Vantman, C. Romero, and M. Vega, “Metformin augments the levels of molecules that regulate the expression of the insulin-dependent glucose transporter GLUT4 in the endometria of hyperinsulinemic PCOS patients,” Hum. Reprod., 28, No. 8, 2235–2244 (2013).
C. Chau-Van, M. Gamba, R. Salvi, R. C. Gaillard, and F. P. Pralong, “Metformin inhibits adenosine 5’-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons,” Endocrinology, 148, 507–511 (2007).
Y. Chen, K. Zhou, R. Wang, Y. Liu, Y. D. Kwak, T. Ma, R. C. Thompson, Y. Zhao, L. Smith, L. Gasparini, Z. Luo, H. Xu, and F. F. Liao, “Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription,” Proc. Natl. Acad. Sci. USA, 106, No. 10, 3907–3912 (2009).
D. Cota, K. Proulx, K. A. Smith, S. C. Kozma, G. Thomas, S. C. Woods, and R. J. Seeley, “Hypothalamic mTOR signaling regulates food intake,” Science, 312, No. 5775, 927–930 (2006).
Y. Dagon, E. Hur, B. Zheng, K. Wellenstein, L. C. Cantley, and B. B. Kahn, “p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake,” Cell Metab., 16, No. 1, 104–112 (2012).
Y. Duan, R. Zhang, M. Zhang, L. Sun, S. Dong, G. Wang, J. Zhang, and Z. Zhao, “Metformin inhibits food intake and neuropeptide Y gene expression in the hypothalamus,” Neural Regen. Res, 8, 2379–2388 (2013).
M. Dulovic, M. Jovanovic, M. Xilouri, L. Stefanis, L. Harhaji-Trajkovic, T. Kravic-Stevovic, V. Paunovic, M. T. Ardah, O. M. El-Agnaf, V. Kostic, I. Markovic, and V. Trajkovic, “The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro,” Neurobiol. Dis., 63, 1–11 (2014).
L. J. Fick, G. H. Fick, and D. D. Belsham, “Palmitate alters the rhythmic expression of molecular clock genes and orexigenic neuropeptide Y mRNA levels within immortalized, hypothalamic neurons,” Biochem. Biophys. Res. Commun., 413, No. 3, 414–419 (2011).
R. J. Ford, M. D. Fullerton, S. L. Pinkosky, E. A. Day, J. W. Scott, J. S. Oakhill, A. L. Bujak, B. K. Smith, J. D. Crane, R. M. Blumer, K. Marcinko, B. E. Kemp, H. C. Gerstein, and G. R. Steinberg, “Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity,” Biochem. J., 468, 125–132 (2015).
M. O. Goodarzi and M. Bryer-Ash, “Metformin revisited: re-evaluation of its properties and role in the pharmacopoeia of modern antidiabetic agents,” Diab. Obes. Metab., 7, No. 6, 654–665 (2005).
S. A. Hawley, M. D. Fullerton, F. A. Ross, J. D. Schertzer, C. Chevtzoff, K. J. Walker, M. W. Peggie, D. Zibrova, K. A. Green, K. J. Mustard, B. E. Kemp, K. Sakamoto, G. R. Steinberg, and D. G. Hardie, “The ancient drug salicylate directly activates AMPactivated protein kinase,” Science, 336, No. 6083, 918–922 (2012).
S. A. Hawley, D. A. Pan, K. J. Mustard, L. Ross, J. Bain, A. M. Edelman, B. G. Frenguelli, and D. G. Hardie, “Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase,” Cell. Metab., 2, 9–19 (2005).
M. Hou, N. Venier, L. Sugar, M. Musquera, M. Pollak, A. Kiss, N. Fleshner, L. Klotz, and V. Venkateswaran, “Protective effect of metformin in CD1 mice placed on a high carbohydrate-high fat diet,” Biochem. Biophys. Res. Commun., 397, 537–542 (2010).
L. Huai, C. Wang, C. Zhang, Q. Li, Y. Chen, Y. Jia, Y. Li, H. Xing, Z. Tian, Q. Rao, M. Wang, and J. Wang, “Metformin induces differentiation in acute promyelocytic leukemia by activating the MEK/ERK signaling pathway,” Biochem. Biophys. Res. Commun., 422, No. 3, 398–404 (2012).
P. Imfeld, M. Bodmer, S. S. Jick, and C. R. Meier, “Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study,” J. Am. Geriatr. Soc., 60, 916–921 (2012).
J. Jin, H. Gu, N. M. Anders, T. Ren, M. Jiang, M. Tao, Q. Peng, M. A. Rudek, and W. Duan, “Metformin protects cells from mutant Huntingtin toxicity through activation of AMPK and modulation of itochondrial dynamics,” Neuromolecular. Med, 18, No. 4, 581–592 (2016).
I. Kawashima, T. Mitsumori, Y. Nozaki, T. Yamamoto, Y. Shobu-Sueki, K. Nakajima, and K. Kirito, “Negative regulation of the LKB1/AMPK pathway by ERK in human acute myeloid leukemia cells,” Exp. Hematol., 43, No. 7, 524–533 (2015).
H. J. Kim, E. Y. Park, M. J. Oh, S. S. Park, K. H. Shin, S. H. Choi, B. G. Chun, and D. H. Kim, “Central administration of metformin into the third ventricle of C57BL/6 mice decreases meal size and number and activates hypothalamic S6 kinase,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 305, R499–R505 (2013).
C. K. Lee, Y. J. Choi, S. Y. Park, J. Y. Kim, K. C. Won, and Y. W. Kim, “Intracerebroventricular injection of metformin induces anorexia in rats,” Diabetes Metab. J., 36, 293–299 (2012).
W. S. Li, J. P. Wen, L. Li, R. X. Sun, J. Wang, Y. X. Xian, C. X. Cao, Y. L. Wang, and Y. Y. Gao, “The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats,” Brain Res., 1444, 11–19 (2012).
A. Malki and A. Youssef, “Antidiabetic drug metformin induces apoptosis in human MCF breast cancer via targeting ERK signaling,” Oncol. Res., 19, No. 6, 275–285 (2011).
T. L. Martin, T. Alquier, K. Asakura, N. Furukawa, F. Preitner, and B. B. Kahn, “Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle,” J. Biol. Chem., 281, No. 28, 18933–18941 (2006).
Y. Matsui, Y. Hirasawa, T. Sugiura, T. Toyoshi, K. Kyuki, and M. Ito, “Metformin reduces body weight gain and improves glucose intolerance in high-fat diet-fed C57BL/6J mice,” Biol. Pharm. Bull., 33, 963–970 (2010).
J. B. McGill, “Pharmacotherapy in type 2 diabetes: a functional schema for drug classification,” Curr. Diabetes Rev., 8, 257–267 (2012).
Y. Minokoshi, T. Alquier, N. Furukawa, Y. B. Kim, A. Lee, B. Xue, J. Mu, F. Foufelle, P. Ferre, M. J. Birnbaum, B. J. Stuck, and B. B. Kahn, “AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus,” Nature, 428, 569–574 (2004).
D. M. Nathan, J. B. Buse, M. B. Davidson, E. Ferrannini, R. R. Holman, R. Sherwin, and B. Zinman, “Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes,” Diabetologia, 52, No. 1, 17–30 (2009).
G. Paolisso, L. Amato, R. Eccellente, A. Gambardella, M. R. Tagliamonte, G. Varricchio, C. Carella, D. Giugliano, and F. D’Onofrio, “Effect of metformin on food intake in obese subjects,” Eur. J. Clin. Invest., 28, 441–446 (1998).
P. Picone, D. Nuzzo, L. Caruana, E. Messina, A. Barera, S. Vasto, and M. Di Carlo, “Metformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF-kB activation: use of insulin to attenuate metformin’s effect,” Biochim. Biophys. Acta, 1853, 1046–1059 (2015).
P. Picone, S. Vilasi, F. Librizzi, M. Contardi, D. Nuzzo, L. Caruana, S. Baldassano, A. Amato, F. Mulè, P. L. San Biagio, D. Giacomazza, and M. Di Carlo, “Biological and biophysics aspects of metformin-induced effects: cortex mitochondrial dysfunction and promotion of toxic amyloid pre-fibrillar aggregates,” Aging (Albany NY), 8, No. 8, 1718–1734 (2016).
Y. Saisho, “Metformin and inflammation: its potential beyond glucose-lowering effect,” Endocr. Metab. Immune Disord. Drug Targets., 15, No. 3, 196–205 (2015).
R. J. Shaw, M. Kosmatka, N. Bardeesy, R. L. Hurley, L. A. Witters, R. A. DePinho, and L. C. Cantley, “The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress,” Proc. Natl. Acad. Sci. USA, 101, No. 10, 3329–3335 (2004).
R. J. Shaw, K. A. Lamia, D. Vasquez, S. H. Koo, N. Bardeesy, R. A. Depinho, M. Montminy, and L. C. Cantley, “The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin,” Science, 310, 1642–1646 (2005).
A. O. Shpakov, K. V. Derkach, and L. M. Berstein, “Brain signaling systems in the type 2 diabetes and metabolic syndrome: promising target to treat and prevent these diseases,” Future Science OA, 1, No. 3, FSO25 (2015), doi: https://doi.org/10.4155/fso.15.23.
Y. Shu, S. A. Sheardown, C. Brown, R. P. Owen, S. Zhang, R. A. Castro, A. G. Ianculescu, L. Yue, J. C. Lo, E. G. Burchard, C. M. Brett, and K. M. Giacomini, “Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action,” J. Clin. Invest., 117, 1422–1431 (2007).
D. L. Smith, Jr., C. F. Elam, Jr., J. A. Mattison, M. A. Lane, G. S. Roth, D. K. Ingram, and D. B. Allison, “Metformin supplementation and life span in Fischer-344 rats,” J. Gerontol. A. Biol. Sci. Med. Sci., 65, 468–474 (2010).
G. R. Steinberg, M. Dandapani, and D. G. Hardie, “AMPK: mediating the metabolic effects of salicylate-based drugs?” Trends Endocrinol. Metab., 24, No. 10, 481–487 (2013).
D. Stevanovic, K. Janjetovic, M. Misirkic, L. Vucicevic, M. Sumarac-Dumanovic, D. Micic, V. Starcevic, and V. Trajkovic, “Intracerebroventricular administration of metformin inhibits ghrelin-induced hypothalamic AMP-kinase signalling and food intake,” Neuroendocrinology, 96, 24–31 (2012).
N. Thangthaeng, M. Rutledge, J. M. Wong, P. H. Vann, M. J. Forster, and N. Sumien, “Metformin impairs spatial memory and visual acuity in old male mice,” Aging Dis., 8, No. 1, 17–30 (2017).
R. P. Vázquez-Manrique, F. Farina, K. Cambon, M. Dolores Sequedo, A. J. Parker, J. M. Millán, A. Weiss, N. Déglon, and C. Neri, “AMPK activation protects from neuronal dysfunction and vulnerability across nematode, cellular and mouse models of Huntington’s disease,” Hum. Mol. Genet., 25, No. 6, 1043–1058 (2016).
B. Viollet and M. Foretz, “Revisiting the mechanisms of metformin action in the liver,” Ann. Endocrinol (Paris), 74, No. 2, 123–129 (2013).
L. Xu and J. D. Ash, “The role of AMPK pathway in neuroprotection,” Adv. Exp. Med. Biol., 854, 425–430 (2016).
W. Ye, E. H. Ramos, B. C. Wong, and D. D. Belsham, “Beneficial effects of metformin and/or salicylate on palmitate-or TNFα-induced neuroinflammatory marker and neuropeptide gene regulation in immortalized NPY/AgRP neurons,” PLoS One, 11, No. 11, e0166973 (2016).
S. Yener, A. Comlekci, B. Akinci, T. Demir, F. Yuksel, M. A. Ozcan, F. Bayraktar, and S. Yesil, “Soluble CD40 ligand, plasminogen activator inhibitor-1 and thrombin-activatable fibrinolysis inhibitor-1-antigen in normotensive type 2 diabetic subjects without diabetic complications. Effects of metformin and rosiglitazone,” Med. Princ. Pract., 18, 266–271 (2009).
J. Zhang, Y. Zhou, C. Chen, F. Yu, Y. Wang, J. Gu, L. Ma, and G. Ho, “ERK1/2 mediates glucose-regulated POMC gene expression in hypothalamic neurons,” J. Mol. Endocrinol., 54, No. 2, 125–135 (2015).
G. Zhou, R. Myers, Y. Li, Y. Chen, X. Shen, J. Fenyk-Melody, M. Wu, J. Ventre, T. Doebber, N. Fujii, N. Musi, M. F. Hirshman, L. J. Goodyear, and D. E. Moller, “Role of AMP-activated protein kinase in mechanism of metformin action,” J. Clin. Invest., 108, No. 8, 1167–1174 (2001).
G. Zhou, J. Yu, A. Wang, S. H. Liu, J. Sinnett-Smith, J. Wu, R. Sanchez, J. Nemunaitis, C. Ricordi, E. Rozengurt, and F. C. Brunicardi, “Metformin restrains pancreatic duodenal homeobox-1 (PDX-1) function by inhibiting ERK signaling in pancreatic ductal adenocarcinoma,” Curr. Mol. Med., 16, 83–90 (2016)
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 103, No. 5, pp. 504–517, May, 2017.
Rights and permissions
About this article
Cite this article
Shpakov, A.O., Derkach, K.V. Molecular Mechanisms of the Effects of Metformin on the Functional Activity of Brain Neurons . Neurosci Behav Physi 48, 969–977 (2018). https://doi.org/10.1007/s11055-018-0657-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-018-0657-6