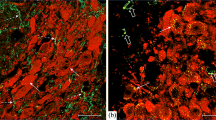
Experiments using C57BL/6J mice 3 h after administration of agouti gene-related peptide (AgRP) fragments 83-132 and 25-51 into the midbrain identified dose-dependent inhibitory effects on dopaminergic neurons. Staining of midbrain sections using an immunohistochemical method with specific antibodies showed that fragment AgRP 83-132 induced significant decreases in tyrosine hydroxylase phosphorylated at serine-40 and serine-31 in neurons. Administration of AgRP fragment 25-51 produced a significant decrease only in tyrosine hydroxylase phosphorylated at serine-31. Studies using high-performance liquid chromatography demonstrated significant decreases in dopamine levels in the striatum after administration of both fragments. This article discusses the mechanisms inducing changes in tyrosine hydroxylase activity in midbrain structures and the actions of AgRP 25-51 via G-protein-independent pathways and particularly the role of the ERK1/2 module of the MAPK kinase cascade.
Similar content being viewed by others
References
H. L. Fields, G. O. Hjelmstad, E. B. Margolis, and S. M. Nicola, “Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement,” Annu. Rev. Neurosci., 30, 289–316 (2007).
G. A. Oganesyan, I. V. Romanova, E. A. Aristakesyan, et al., “The dopaminergic system of the telencephalo-diencephalic areas of the vertebrate brain in the organization of the sleep–waking cycle,” Neurosci. Behav. Physiol., 39, No. 8, 805–817 (2008).
C. R. Gerfen, “Basal ganglia,” in: The Rat Nervous System, Elsevier, USA (2004), 3rd ed., pp. 455–508.
M. V. Ugryumov, “Traditional views of neurodegenerative diseases,” in: Neurodegenerative Diseases: Basic and Applied Aspects, Nauka, Moscow (2010), pp. 8–35.
S. C. Daubner, T. Le, and S. Wang, “Tyrosine hydroxylase and regulation of dopamine synthesis,” Arch. Biochem. Biophys., 508, No. 1, 1–12 (2011).
M. M. Ollmann, B. D. Wilson, Y. K. Yang, et al., “Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein,” Science, 278, 135–138 (1997).
D. Bagnol, X. Y. Lu, C. B. Kaelin, et al., “Anatomy of an endogenous antagonist: relationship between agouti-related protein and proopiomelanocortin in brain,” J. Neurosci., 19, 1–7 (1999).
J. W. Creemers, L. E. Pritchard, A. Gyte, et al., “Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP) 83-132: interaction between AGRP83-132 and melanocortin receptors cannot be influenced by syndecan-3,” Endocrinology, 147, No. 4, 1621–1631 (2006).
M. W. Schwartz and G. J. Morton, “Obesity: keeping hunger at bay,” Nature, 418, 595–597 (2002).
Y. K. Yang, M. Ollmann, B. Wilson, et al., “Effects of recombinant agouti-signaling protein on melanocortin action,” Mol. Endocrinol., 11, 274–280 (1997).
R. D. Cone, “Anatomy and regulation of the central melanocortin system,” Nat. Neurosci., 8, No. 5, 571–578 (2005).
M. Lee and S. L. Wardlaw, “The central melanocortin system and the regulation of energy balance,” Front. Biosci., 12, 3994–4010 (2007).
D. J. Marsh, G. I. Miura, K. A. Yagaloff, et al., “Effects of neuropeptide Y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues,” Brain Res., 848, No. 1, 66–77 (1999).
V. Tolle and M. J. Low, “In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin- deficient mice,” Diabetes, 57, No. 1, 86–94 (2008).
K. Goto, A. Inui, Y. Takimoto, et al., “Acute intracerebroventricular administration of carboxyl-terminal fragments of agouti-related pep-tide produces a long-term decrease in energy expenditure in rats,” Int. J. Mol. Med., 12, 379–383 (2003).
L. E. Pritchard and A. White, “Agouti-related protein: more than a melanocortin-4 receptor antagonist?” Peptides, 26, No. 10, 1759–1770 (2005).
A. L. Mikhrina and I. V. Romanova, “The role of AGRP in regulating dopaminergic neurons in the brain,” Neurosci. Behav. Physiol., 45, No. 5, 536–541 (2015).
R. N. Lippert, K. L. J. Ellacott, and R. D. Cone, “Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice,” Endocrinology, 155, No. 5, 1718–1727 (2014).
G. Paxinos and K. B. J. Franklin, The Mouse Brain in Stereotaxic Coordinates, Academic Press (2001), ISBN 0-12-547636-1, CDROM, www.academicpress.com.
K. L. Lambertsen, J. B. Gramsbergen, M. Sivasaravanaparan, et al., “Genetic KCa3.1-Deficiency produces locomotor hyperactivity and alterations in cerebral monoamine levels,” PLoS One, 7, No. 10,1–15 (2012).
I. N. Krasnova, E. R. Bychkov, V. I. Lioudyno, et al., “Intracerebroventricular administration of substance P increases dopamine content in the brain of 6-hydroxydopamine lesioned rats,” Neuroscience, 95, No. 1, 113–117 (2000).
A. L. Mikhrina, M. V. Chernyshev, E. V. Mikhailova, et al., “Involvement of agouti-like peptide in the control of movement activity,” Ros. Fiziol. Zh., 104, No. 7, 769–779 (2018).
A. C. Rapraeger, “Syndecan-regulated receptor signaling,” J. Cell Biol., 149, No. 5, 995–998 (2000).
J. W. Haycock, N. G. Ahn, M. H. Cobb, and E. G. Krebs, “ERK1 and ERK2, two microtubule-associated protein kinases, mediate the phosphorylation of tyrosine hydroxylase at serine 31 in situ,” Proc. Natl. Acad. Sci. USA, 89, 2365–2369 (1992).
E. Savontaus, I. M. Conwell, and S. L. Wardlaw, “Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats,” Brain Res., 958, No. 1, 130–138 (2002).
I. V. Romanova, K. V. Derkach, A. L. Mikhrina, et al., “The leptin, dopamine and serotonin receptors in hypothalamic POMC-neurons of normal and obese rodents,” Neurochem. Res., 43, No. 4, 821–837 (2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 104, No. 12, pp. 1456–1466, December, 2018.
Rights and permissions
About this article
Cite this article
Mikhrina, A.L., Saveleva, L.O., Alekseeva, O.S. et al. Effects of Active Fragments AgRP 83-132 and 25-51 on Dopamine Biosynthesis in the Brain. Neurosci Behav Physi 50, 367–373 (2020). https://doi.org/10.1007/s11055-020-00908-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-00908-z