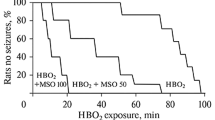
Respiration of hyperbaric oxygen (HBO2) suppresses the synthesis of γ-aminobutyric acid (GABA) in the brain, leading to weakening of inhibitory GABAergic neurotransmission and the development of convulsive syndrome of the epileptic seizure type. We report here our testing of the hypothesis that inhibition of GABA transporters might compensate for insuffi ciency of inhibitory transmitter synthesis, strengthen GABAergic transmission, and weaken or prevent the development of oxygen-induced convulsions. The development of convulsions was studied in conscious rats in a barochamber containing oxygen at a pressure of 5 atm (absolute atmospheres) after prior intracerebroventricular administration of drugs inhibiting selective neuronal (NO-711) or nonselective neuronal/glial GABA transporters (nipecotic acid). Studies in a separate group of rats measured GABA in the striatum by microdialysis with liquid chromatography. These experiments showed that inhibition of neuronal and glial GABA transporters increases the level of GABA in the brain and weakens the development of oxygen-induced convulsions. A more effective anticonvulsant effect was seen after intracerebroventricular administration of the nonselective inhibitor of GABA transporters. These data provide evidence that blockade of the functions of neuronal and glial GABA transporters increases the GABA level in the brain and weakens the development of convulsive syndrome in HBO2. The anticonvulsant effects of the inhibitors used here appear to result from strengthening of GABA-mediated synaptic and extrasynaptic neurotransmission by hyperbaric hyperoxia. Inhibition of GABA transporters may constitute a potential direction for the development of effective methods of preventing oxygen-induced convulsions.
Similar content being viewed by others
References
G. L. Zal’tsman, “Stages of formation of oxygen-induced epilepsy and the functional state of the nervous system,” in: Hyperbaric Epilepsy and Narcosis, G. L. Zal’tsman (ed.), Nauka, Leningrad (1968), pp. 129–136.
J. D. Balentine, Pathology of Oxygen Toxicity, Academic Press, New York (1982).
V. I. Arsen’eva and A. I. Selivra, “Bioelectric activity of the peripheral part of sympathetic nervous system during oxygen-induced epilepsy,” in: Hyperbaric Epilepsy and Narcosis, G. L. Zal’tsman (ed.), Nauka, Leningrad (1968), pp. 70–78.
A. I. Selivra, Hyperbaric Oxygenation. Physiological Mechanisms of the Central Nervous System Responses to Hyperoxia, Nauka, Leningrad (1983).
J. B. Dean, D. K. Mulkey, R. A. Henderson, et al., “Hyperoxia, reactive oxygen species, and hyperventilation: Oxygen sensitivity of brain stem neurons,” J. Appl. Physiol., 96, 784–791 (2004).
J. B. Dean, D. K. Mulkey, A. J. Garcia, et al., “Neuronal sensitivity to hyperoxia, hypercapnia, and inert gases at hyperbaric pressures,” J. Appl. Physiol., 95, 883–909 (2003).
I. T. Demchenko and C. A. Piantadosi, “Nitric oxide amplifi es the excitatory to inhibitory neurotransmitter imbalance accelerating oxygen seizures,” Undersea Hyperb. Med., 33, No. 3, 169–174 (2006).
G. V. Shcherbakova, “Glutamate decarboxylase activity and γ-aminobutyric acid content in rat brain at different functional states caused by high oxygen pressure,” Dokl. Akad. Nauk. SSSR, 146, No. 5, 1213–1215 (1962).
J. D. Wood and W. J. Watson, “Protective action of gamma-aminobutyric acid against oxygen toxicity,” Nature, 195, 296 (1962).
A. A. Krichevskaya, V. S. Shugalei, L. A. Shcherbina, and G. G. Ermolenko, “γ-Aminobutyric acid contents and glutamate decarboxylase activity in the brains of rats in hyperbaric oxygen and the protective action of urea,” Vopr. Med. Khim., 20, No. 3, 294–298 (1974).
A. K. Singh, and E. W. Banister, “Effect of 6-hydroxydopamine on brain and blood catecholamine, ammonia, and amino acid metabolism in rats subjected to high pressure oxygen induced convulsions,” Can. J. Physiol. Pharmacol., 56, No. 2, 334–336 (1978).
P. Mialon, R. Gibey, J. C. Bigot, and L. Barthelemy, “Changes in striatal and cortical amino acid and ammonia levels of rat brain after one hyperbaric oxygen-induced seizures,” Aviat. Space Environ. Med., 63, No. 4, 287–291 (1992).
I. T. Demchenko, A. E. Boso, A. R. Whorton, and C. A. Piantadosi, “Nitric oxide production is enhanced in rat brain before oxygen-induced convulsions,” Brain Res., 917, No. 2, 253–261 (2001).
S. Zhang, Y. Takeda, S. Hagioka, et al., “Measurement of GABA and glutamate in vivo levels with high sensitivity and frequency,” Brain Res. Protoc., 14, No. 2, 61–66 (2005).
H. G. Gasier, I. T. Demchenko, L. G. Tatro, and C. A. Piantadosi, “S-nitrosylation of GAD65 is implicated in decreased GAD activity and oxygen-induced seizures,” Neurosci. Lett., 653, 283–287 (2017).
D. P. D’Agostino, R. W. Putnam, and J. B. Dean, “Superoxide (O2–) production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen,” J. Neurophysiol., 98, 1030–1041 (2007).
S. R. Thom, V. Bhopale, D. Fisher, et al., “Stimulation of nitric oxide synthase in cerebral cortex due to elevated partial pressures of oxygen: An oxidative stress response,” J. Neurobiol., 51, 85–100 (2002).
N. Gould, P. T. Doulias, M. Tenopoulou, et al., “Regulation of protein function and signaling by reversible cysteine S-nitrosylation,” J. Biol. Chem., 288, No. 37, 26473–26479 (2013).
D. N. Atochin, I. T. Demchenko, J. Astern, et al., “Contributions of endothelial and neuronal nitric oxide synthases to cerebrovascular responses to hyperoxia,” J. Cereb. Blood Flow Metab., 23, 1219–1226 (2003).
G. B. Richerson and Y. Wu, “Dynamic equilibrium of neurotransmitter transporter. Not just for reuptake anymore,” J. Neurophysiol., 90, 1363–1374 (2003).
A. V. Sem’yanov, Diffuse extrasynaptic neurotransmission by glutamate and GABA,” Zh. Vyssh. Nerv. Deyat., 54, No. 1, 66–82 (2003).
M. van der Zeyden, W. H. Oldenziel, K. Rea, et al., “Microdialysis of GABA and glutamate: Analysis, interpretation and comparison with microsensors,” Pharmacol. Biochem. Behav., 90, 135–147 (2008).
A. Del Arco, G. Segovia, R. Fuxe, and F. Mora, “Changes in dialysate concentrations of glutamate and GABA in the brain: An index of volume transmission mediated actions?” J. Neurochem., 85, 23–33 (2003).
T. Tzuk-Shina, N. Bitterman, and D. Harel, “The effect of vigabatrin on central nervous system oxygen toxicity in rats,” Eur. J. Pharmacol., 202, No. 2, 171–175 (1991).
A. A. Hall, C. Young, M. Bodo, and R. T. Mahon, “Vigabatrin prevents seizure in swine subjected to hyperbaric hyperoxia,” J. Appl. Physiol., 115, No. 6, 861–867 (2013).
I. T. Demchenko, S. Y. Zhilyaev, A. N. Moskvin, et al., “Antiepileptic drugs prevent seizures in hyperbaric oxygen: A novel model of epileptiform activity,” Brain Res., 1657, 347–354 (2017).
F. Kersante, S. Rowley, I. Pavlov, et al., “A functional role for both γ-aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus,” J. Physiol., 591, No. 10, 2429–2441 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 105, No. 4, pp. 510–519, April, 2019.
Rights and permissions
About this article
Cite this article
Moskvin, A.N., Platonova, T.P., Zhilyaev, S.Y. et al. Blockade Of γ-Aminobutyric Acid Transporters In Brain Synapses Protects Against Hyperbaric Oxygen-Induced Convulsions. Neurosci Behav Physi 50, 505–510 (2020). https://doi.org/10.1007/s11055-020-00930-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-00930-1