Abstract
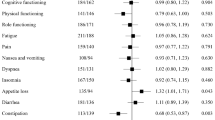
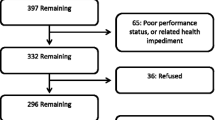
In recurrent glioblastoma, health-related quality of life (HRQL) is a crucial trial endpoint. We examined HRQL outcomes as a secondary endpoint for patients in the CABARET randomized phase 2 trial. 122 patients were randomly allocated to bevacizumab monotherapy or bevacizumab plus carboplatin. We calculated change scores from baseline for each HRQL measure on the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and the Brain Cancer Module (QLQ-BN20), together with time to deterioration in HRQL, and the proportion of participants with clinically meaningful improvements in specific disease-related symptoms. At baseline, 117 of 122 randomized patients (96%) attempted questionnaires. Questionnaire participation rates were >90% for patients continuing on treatment, however at the end-of-treatment visit only 72 (64% of eligible participants) returned a form. There were no differences between arms in change scores over the treatment period. Time to ≥10 point deterioration in scores from baseline was also similar between arms. HRQL deterioration occurred largely before progression for the domains tested, but scores in HRQL domains specifically relevant to symptoms of recurrent glioblastoma also improved for about 50% of patients with symptoms at baseline. Neither detrimental nor beneficial effects on HRQL were seen with carboplatin added to bevacizumab, with a proportion of patients on both arms experiencing symptomatic benefit. Given the reduced questionnaire completion at end of treatment, time to HRQL deterioration is a feasible and robust clinical trial endpoint in this patient population. Clinical trials registration number: ACTRN12610000915055.


Similar content being viewed by others
References
Field KM, Simes J, Nowak AK, Cher L, Wheeler H, Hovey EJ, Brown CS, Barnes EH, Sawkins K, Livingstone A, Freilich R, Phal PM, Fitt G, Cabaret COGNO investigators, Rosenthal MA (2015) Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro-Oncology 17:1504–1513. doi:10.1093/neuonc/nov104
Hovey EJ, Field KM, Rosenthal M, Nowak AK, Cher L, Wheeler H, Barnes E, Phal P, Livingstone A, Sawkins K, Simes J, COGNO CABARET Investigators (2016) Continuing or ceasing bevacizumab at disease progression: results from the CABARET study, a prospective randomized phase II trial in patients with recurrent glioblastoma. Neuro-Oncol Pract (in press)
Fayers P, Bottomley A, EORTC Quality of Life Group, Quality of Life Unit (2002) Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer 38:S125–133
Taphoorn MJ, Claassens L, Aaronson NK, Coens C, Mauer M, Osoba D, Stupp R, Mirimanoff RO, van den Bent MJ, Bottomley A, EORTC Quality of Life Group, and Brain Cancer, NCIC, and Radiotherapy Groups (2010) An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer 46:1033–1040. doi:10.1016/j.ejca.2010.01.012
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, & on behalf of the EORTC Quality of Life Group (2001) EORTC QLQ-C30 Scoring manual, 3rd edn. European Organisation for Research and Treatment of Cancer, Brussels
Osoba D, Bezjak A, Brundage M, Zee B, Tu D, Pater J, Quality of Life Committee of the NC (2005) Analysis and interpretation of health-related quality-of-life data from clinical trials: basic approach of The National Cancer Institute of Canada Clinical Trials Group. Eur J Cancer 41:280–287. doi:10.1016/j.ejca.2004.10.017
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139–144
Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, Gundy C, Koller M, Petersen MA, Sprangers MAG, on behalf of the EORTC Quality of Life Group (2008) EORTC QLQ-C30 reference values. EORTC, Brussels
Brown PD, Jensen AW, Felten SJ, Ballman KV, Schaefer PL, Jaeckle KA, Cerhan JH, Buckner JC (2006) Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J Clin Oncol 24:5427–5433. doi:10.1200/jco.2006.08.5605
Meyers CA, Hess KR (2003) Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. NeuroOncology 5:89–95. doi:10.1215/S1522-8517-02-00026-1
Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE, DeAngelis LM, Lassman AB (2009) Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 73:1200–1206. doi:10.1212/WNL.0b013e3181bc0184
Dirven L, van den Bent MJ, Bottomley A, van der Meer N, van der Holt B, Vos MJ, Walenkamp AM, Beerepoot LV, Hanse MC, Reijneveld JC, Otten A, de Vos FY, Smits M, Bromberg JE, Taal W, Taphoorn MJ (2015) The impact of bevacizumab on health-related quality of life in patients treated for recurrent glioblastoma: results of the randomised controlled phase 2 BELOB trial. Eur J Cancer 51:1321–1330. doi:10.1016/j.ejca.2015.03.025
Taphoorn MJ, Henriksson R, Bottomley A, Cloughesy T, Wick W, Mason WP, Saran F, Nishikawa R, Hilton M, Theodore-Oklota C, Ravelo A, Chinot OL (2015) Health-related quality of life in a randomized phase iii study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol 33:2166–2175. doi:10.1200/JCO.2014.60.3217
Armstrong TS, Won M, Wefel JS, Gilbert MR, Pugh SL, Brachman D, Komaki R, Crocker IR, Robins I, Lee J, Mehta MP, Wendland MM (2013) Comparative impact of treatment on patient reported outcomes in patients with glioblastoma enrolled in RTOG 0825. ASCO Annual Meeting. Journal of Clinical Oncology, Chicago, p 2003
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708. doi:10.1056/NEJMoa1308573
Field KM, Jordan JT, Wen PY, Rosenthal MA, Reardon DA (2015) Bevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversies. Cancer 121:997–1007. doi:10.1002/cncr.28935
Wick W, Brandes AA (2015) Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: The EORTC 26101 trial. Neuro-Oncology 17:v1
Acknowledgements
This trial was conducted under the auspices of the Cooperative Trials Group for Neuro-Oncology (COGNO), coordinated at the NHMRC Clinical Trials Centre, University of Sydney, supported by Roche Products Pty Limited (Australia). Professor King is supported by the Australian Government through Cancer Australia.
Funding
This study was funded by Roche Products, Pty Limited (Australia) with support also from NHMRC Program Grant 1037786 to the NHMRC Clinical Trials Centre, and Cancer Australia Support for Cancer Clinical Trials Grant and CINSW Cooperative Clinical Trials Grant to COGNO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KMF was funded through the University of Melbourne Stella Mary Langford Scholarship and the Royal Melbourne Hospital Research Medal and Watt-Geyer Memorial Research Fund. She has received travel grants from Roche to attend conferences. EJH has had consulting or advisory roles for Bayer, Janssen Oncology, Pfizer, and Roche, and has received travel grants from GlaxoSmithKline and Sanofi. MAR has been on a Roche Advisory Board. LC has received honoraria from and has had consulting or advisory roles for Roche Pharma AG, and has received institutional research funding from Celldex, Lilly, Merck, and Roche. AKN has had consulting or advisory roles for Boehringer Ingelheim and Roche and has received research funding from Boehringer Ingelheim. JS and KS have received institutional research funding from Roche through the Clinical Trials Centre. DE, EB and HW declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving animal participants
This article does not contain any studies with animals performed by any of the authors.
Additional information
Trial registration
Clinical trials registration number: ACTRN12610000915055.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Field, K.M., King, M.T., Simes, J. et al. Health-related quality of life outcomes from CABARET: a randomized phase 2 trial of carboplatin and bevacizumab in recurrent glioblastoma. J Neurooncol 133, 623–631 (2017). https://doi.org/10.1007/s11060-017-2479-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2479-8