Abstract
The loss of nigral dopaminergic (DA) neurons is the disease-defining pathological change responsible for progressive motor dysfunction in Parkinson’s disease. In this study, we sought to establish a culture method for adult rat tyrosine hydroxylase (TH)-immunoreactive DA neurons. In this context, we investigated the role of fibroblast growth factor 2 (FGF2), brain-derived neurotrophic factor (BDNF), transforming growth factor-β3 (TGF-β3), glial-derived neurotrophic factor (GDNF) and dibutyryl-cyclic AMP (dbcAMP) in these cultures. Culturing in the presence of FGF2, BDNF and GDNF enhanced the survival of DA neurons by 15-fold and promoted neurite growth. In contrast, dbcAMP promoted neurite growth in all neurons but did not enhance DA cell survival. This study demonstrates that long-term cultures of DA neurons can be established from the mature rat brain and that survival and regeneration of DA neurons can be manipulated by epigenetic factors such as growth factors and intracellular cAMP pathways.








Similar content being viewed by others
References
Woolf CJ (2001) Turbocharging neurons for growth: accelerating regeneration in the adult CNS. Nat Neurosci 4:7–9
Sapieha PS, Duplan L, Uetani N et al (2005) Receptor protein tyrosine phosphatase sigma inhibits axon regrowth in the adult injured CNS. Mol Cell Neurosci 28:625–635
Smith PD, Sun F, Park KK et al (2009) SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 64:617–623
Chesselet MF (2003) Dopamine and Parkinson’s disease: is the killer in the house? Mol. Psychiatry. 8:369–370
Uversky VN, Eliezer D (2009) Biophysics of Parkinson’s disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci 10:483–499
Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535
Liu J, Head E, Gharib AM et al (2002) Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci USA 99:2356–2361
Aliev G, Palacios HH, Walrafen B et al (2009) Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int J Biochem Cell Biol 41:1989–2004
Terman A, Gustafsson B, Brunk UT (2006) Mitochondrial damage and intralysosomal degradation in cellular aging. Mol Aspects Med 27:471–482
Terman A, Kurz T, Navratil M et al (2010) Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal 12:503–535
Bronzetti E, Felici L, Ferrante F et al (1988) Age-related changes of the metabolic profile of rat cerebellar cortex: enzyme histochemical study. Mech Ageing Dev 44:277–286
Rogers SW, Gahring LC, Collins AC et al (1998) Age-related changes in neuronal nicotinic acetylcholine receptor subunit alpha4 expression are modified by long-term nicotine administration. J Neurosci 18:4825–4832
Pascale A, Govoni S, Battaini F (1998) Age-related alteration of PKC, a key enzyme in memory processes: physiological and pathological examples. Mol Neurobiol 16:49–62
Zhou W, Schaack J, Zawada WM et al (2002) Overexpression of human alpha-synuclein causes dopamine neuron death in primary human mesencephalic culture. Brain Res 926:42–50
Ren D, Miller JD (2003) Primary cell culture of suprachiasmatic nucleus. Brain Res Bull 61:547–553
Martinoia S, Bonzano L, Chiappalone M et al (2005) In vitro cortical neuronal networks as a new high-sensitive system for biosensing applications. Biosens Bioelectron 20:2071–2078
Ganser C, Papazoglou A, Just L et al (2010) Neuroprotective effects of erythropoietin on 6-hydroxydopamine-treated ventral mesencephalic dopamine-rich cultures. Exp Cell Res 316:737–746
Prang P, Del Turco D, Kapfhammer JP (2001) Regeneration of entorhinal fibers in mouse slice cultures is age dependent and can be stimulated by NT-4, GDNF, and modulators of G-proteins and protein kinase C. Exp Neurol 169:135–147
Blackmore M, Letourneau PC (2006) Changes within maturing neurons limit axonal regeneration in the developing spinal cord. J Neurobiol 66:348–360
Wanner IB, Deik A, Torres M et al (2008) A new in vitro model of the glial scar inhibits axon growth. Glia 56:1691–1709
Schwab ME (2002) Repairing the injured spinal cord. Science 295:1029–1031
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146–156
Spencer T, Domeniconi M, Cao Z et al (2003) New roles for old proteins in adult CNS axonal regeneration. Curr Opin Neurobiol 13:133–139
Lu P, Tuszynski MH (2008) Growth factors and combinatorial therapies for CNS regeneration. Exp Neurol 209:313–320
Brewer GJ (1997) Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods 71:143–155
Evans J, Sumners C, Moore J et al (2002) Characterization of mitotic neurons derived from adult rat hypothalamus and brain stem. J Neurophysiol 87:1076–1085
Majd S, Zarifkar A, Rastegar K et al (2008) Different fibrillar Abeta 1–42 concentrations induce adult hippocampal neurons to reenter various phases of the cell cycle. Brain Res 1218:224–229
Thoenen H (1995) Neurotrophins and neuronal plasticity. Science 270:593–598
Song XY, Li F, Zhang FH et al (2008) Peripherally-derived BDNF promotes regeneration of ascending sensory neurons after spinal cord injury. PLoS One 3:e1707
Gao H, Qiao X, Hefti F et al (1997) Elevated mRNA expression of brain-derived neurotrophic factor in retinal ganglion cell layer after optic nerve injury. Invest Ophthalmol Vis Sci 38:1840–1847
Loeliger MM, Briscoe T, Rees SM (2008) BDNF increases survival of retinal dopaminergic neurons after prenatal compromise. Invest Ophthalmol Vis Sci 49:1282–1289
Spenger C, Hyman C, Studer L et al (1995) Effects of BDNF on dopaminergic, serotonergic, and GABAergic neurons in cultures of human fetal ventral mesencephalon. Exp Neurol 133:50–63
Lin LF, Doherty DH, Lile JD et al (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132
Nicole O, Ali C, Docagne F et al (2001) Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J Neurosci 21:3024–3033
Rahhal B, Heermann S, Ferdinand A et al (2009) In vivo requirement of TGF-beta/GDNF cooperativity in mouse development: focus on the neurotrophic hypothesis. Int J Dev Neurosci 27:97–102
Schaar DG, Sieber BA, Dreyfus CF et al (1993) Regional and cell-specific expression of GDNF in rat brain. Exp Neurol 124:368–371
Roussa E, von Bohlen und Halbach O, Krieglstein K (2009) TGF-beta in dopamine neuron development, maintenance and neuroprotection. Adv Exp Med Biol 651:81–90
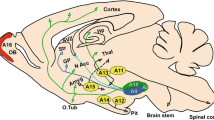
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier academic press, San Diego
Majd S, Rastegar K, Zarifkar A et al (2007) Fibrillar beta-amyloid (Abeta) (1–42) elevates extracellular Abeta in cultured hippocampal neurons of adult rats. Brain Res 1185:321–327
Majd S, Zarifkar A, Rastegar K et al (2008) Culturing adult rat hippocampal neurons with long-interval changing media. Iran Biomed J 12:101–107
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Hyman C, Hofer M, Barde YA et al (1991) BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350:230–232
Engele J, Franke B (1996) Effects of glial cell line-derived neurotrophic factor (GDNF) on dopaminergic neurons require concurrent activation of cAMP-dependent signaling pathways. Cell Tissue Res 286:235–240
Kawano H, Li HP, Sango K et al (2005) Inhibition of collagen synthesis overrides the age-related failure of regeneration of nigrostriatal dopaminergic axons. J Neurosci Res 80:191–202
Goldberg JL (2004) Intrinsic neuronal regulation of axon and dendrite growth. Curr Opin Neurobiol 14:551–557
Kloos AD, Muller KJ, Modney BK (2007) Atypical embryonic synapses fail to regenerate in adulthood. J Comp Neurol 505:404–411
Cohen-Cory S, Kidane AH, Shirkey NJ et al (2009) Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol 70:271–288
Borghesani PR, Peyrin JM, Klein R et al (2002) BDNF stimulates migration of cerebellar granule cells. Development 129:1435–1442
Liu X, Grishanin RN, Tolwani RJ et al (2007) Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci 27:7256–7267
Zeng F, Watson RP, Nash MS (2010) Glial cell-derived neurotrophic factor enhances synaptic communication and 5-hydroxytryptamine 3a receptor expression in enteric neurons. Gastroenterology 138:1491–1501
Alberch J, Perez-Navarro E, Canals JM (2002) Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington’s disease. Brain Res Bull 57:817–822
Alexi T, Borlongan CV, Faull RL et al (2000) Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Prog Neurobiol 60:409–470
Sharma HS (2010) Selected combination of neurotrophins potentiate neuroprotection and functional recovery following spinal cord injury in the rat. Acta Neurochir Suppl 106:295–300
Deister C, Schmidt CE (2006) Optimizing neurotrophic factor combinations for neurite outgrowth. J Neural Eng 3:172–179
Logan A, Ahmed Z, Baird A et al (2006) Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain 129:490–502
Wright DE, Snider WD (1995) Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol 351:329–338
Numan S, Seroogy KB (1999) Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: a double-label in situ hybridization study. J Comp Neurol 403:295–308
Walker DG, Beach TG, Xu R et al (1998) Expression of the proto-oncogene Ret, a component of the GDNF receptor complex, persists in human substantia nigra neurons in Parkinson’s disease. Brain Res 792:207–217
He SM, Zhao ZW, Zhao L et al (2008) Nigrostriatal projecting neurons express GDNF receptor subunit RET in adult rats. Acta Neurobiol Exp (Wars) 68:347–353
Glazner GW, Mu X, Springer JE (1998) Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol 391:42–49
Trupp M, Belluardo N, Funakoshi H et al (1997) Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci 17:3554–3567
Tsuzuki T, Takahashi M, Asai N et al (1995) Spatial and temporal expression of the ret proto-oncogene product in embryonic, infant and adult rat tissues. Oncogene 10:191–198
Nosrat CA, Tomac A, Hoffer BJ et al (1997) Cellular and developmental patterns of expression of Ret and glial cell line-derived neurotrophic factor receptor alpha mRNAs. Exp Brain Res 115:410–422
Soderstrom S, Bengtsson H, Ebendal T (1996) Expression of serine/threonine kinase receptors including the bone morphogenetic factor type II receptor in the developing and adult rat brain. Cell Tissue Res 286:269–279
Charytoniuk DA, Traiffort E, Pinard E et al (2000) Distribution of bone morphogenetic protein and bone morphogenetic protein receptor transcripts in the rodent nervous system and up-regulation of bone morphogenetic protein receptor type II in hippocampal dentate gyrus in a rat model of global cerebral ischemia. Neuroscience 100:33–43
Remenyi J, Hunter CJ, Cole C et al (2010) Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J 428:281–291
Cui Q, Yip HK, Zhao RC et al (2003) Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci 22:49–61
Meyer-Franke A, Kaplan MR, Pfrieger FW et al (1995) Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 15:805–819
Shen S, Wiemelt AP, McMorris FA et al (1999) Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron 23:285–295
Rydel RE, Greene LA (1988) cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc Natl Acad Sci USA 85:1257–1261
Li M, Wang X, Meintzer MK et al (2000) Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol 20:9356–9363
Acknowledgments
We would like to thank Jim Massalas for technical assistance. JD is an NHMRC Research Fellow. The funding for the conduct of the research was obtained from the NHMRC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Majd, S., Smardencas, A., Parish, C.L. et al. Development of an In Vitro Model to Evaluate the Regenerative Capacity of Adult Brain-Derived Tyrosine Hydroxylase-Expressing Dopaminergic Neurons. Neurochem Res 36, 967–977 (2011). https://doi.org/10.1007/s11064-011-0435-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0435-0