Abstract
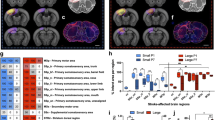
While astrocytes are recognised to play a central role in repair processes following stroke, at this stage we do not have a clear understanding of how these cells are engaged during the chronic recovery phase. Accordingly, the principal aim of this study was to undertake a quantitative multi-regional investigation of astrocytes throughout the recovery process. Specifically, we have induced experimental vascular occlusion using cold-light photothrombotic occlusion of the somatosensory/motor cortex in adult male C57B6 mice. Four weeks following occlusion we collected, processed, and immunolabelled tissue using an antibody directed at the glial fibrillary acidic protein (GFAP), an astrocyte specific cytoskeletal protein marker. We investigated GFAP changes in 13 regions in both the contra- and ipsi-lateral hemispheres from control and occluded animals. Specifically, we examined the infra-limbic (A24a), pre-limbic (A25), anterior cingulate (A32), motor (M1 and M2) cortices, the forceps minor fibre tract, as well the shell of the accumbens, thalamus, cingulate cortex (A29c), hippocampus (CA1-3) and lateral hypothalamus. Tissue from occluded animals was compared against sham treated controls. We have identified that the focal occlusion produced significant astrogliosis (p < 0.05), as defined by a marked elevation in GFAP expression, within all 13 sites assessed within the ipsilateral (lesioned) hemisphere. We further observed significant increases in GFAP expression (p < 0.05) in 9 of the 13 contralesional sites examined. This work underscores that both the ipsilateral and contralesional hemispheres, at sites distal to the infarct, are very active many weeks after the initial occlusion, a finding that potentially has significant implications for understanding and improving the regeneration of the damaged brain.




Similar content being viewed by others
References
Nudo RJ (1997) Remodeling of cortical motor representations after stroke: implications for recovery from brain damage. Mol Psychiatry 2:188–191
Nudo RJ (1999) Recovery after damage to motor cortical areas. Curr Opin Neurobiol 9:740–747
Nudo RJ (2006) Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol 16:638–644
Nudo RJ (2007) Postinfarct cortical plasticity and behavioral recovery. Stroke 38:840–845
Teasell R, Bayona NA, Bitensky J (2005) Plasticity and reorganization of the brain post stroke. Top Stroke Rehabil 12:11–26
Pekna M, Pekny M, Nilsson M (2012) Modulation of neural plasticity as a basis for stroke rehabilitation. Stroke 43:2819–2828
Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Jacob W, Lent R, Herculano-Houzel S (2009) Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513:532–541
Turner RC, Dodson SC, Rosen CL, Huber JD (2013) The science of cerebral ischemia and the quest for neuroprotection: navigating past failure to future success a review. J Neurosurg 118:1072–1085
Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28:468–481
Pekny M, Wilhelmsson U, Pekna M (2014) The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 565:30–38
Liu Z, Li Y, Cui Y, Roberts C, Lu M, Wilhelmsson U, Pekny M, Chopp M (2014) Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia 62:2022–2033. doi:10.1002/glia.22723
Schmidt-Kastner R, Wietasch K, Weigel H, Eysel UT (1993) Immunohistochemical staining for glial fibrillary acidic protein (GFAP) after deafferentation or ischemic infarction in rat visual system: features of reactive and damaged astrocytes. Int J Dev Neurosci 11:157–174
Schroeter M, Schiene K, Kraemer M, Hagemann G, Weigel H, Eysel UT, Witte OW, Stoll G (1995) Astroglial responses in photochemically induced focal ischemia of the rat cortex. Exp Brain Res 106:1–6
Li HL, Zhang NN, Lin HY, Yu Y, Cai QY, Ma LX, Ding SH (2014) Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis-induced ischemia in adult mice. BMC Neurosci 15:58. doi:10.1186/1471-2202-15-58
Nowicka D, Rogozinska K, Aleksy M, Witte OW, Skangiel-Kramska J (2008) Spatiotemporal dynamics of astroglial and microglial responses after photothrombotic stroke in the rat brain. Acta Neurobiol Exp 68:155–168
Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, Schwab S, Sommer C (2004) Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke 35:992–997
Haupt C, Witte OW, Frahm C (2007) Up-regulation of Connexin43 in the glial scar following photothrombotic ischemic injury. Mol Cell Neurosci 35:89–99
Bidmon HJ, Jancsik V, Schleicher A, Hagemann G, Witte OW, Woodhams P, Zilles K (1998) Structural alterations and changes in cytoskeletal proteins and proteoglycans after focal cortical ischemia. Neuroscience 82:397–420
Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, Walker FR (2013) Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol 126:75–91
Paxinos G, Franklin K (2001) The mouse brain in stereotaxic coordinates. Elsevier, San Diego
Wagner DC, Scheibe J, Glocke I, Weise G, Deten A, Boltze J, Kranz A (2013) Object-based analysis of astroglial reaction and astrocyte subtype morphology after ischemic brain injury. Acta Neurobiol Exp 73:79–87
Johansen-Berg H, Rushworth MFS, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM (2002) The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA 99:14518–14523
Crofts JJ, Higham DJ, Bosnell R, Jbabdi S, Matthews PM, Behrens TEJ, Johansen-Berg H (2011) Network analysis detects changes in the contralesional hemisphere following stroke. Neuroimage 54:161–169
Takatsuru Y, Eto K, Kaneko R, Masuda H, Shimokawa N, Koibuchi N, Nabekura J (2013) Critical role of the astrocyte for functional remodeling in contralateral hemisphere of somatosensory cortex after stroke. J Neurosci 33:4683–4692
Gillespie DC, Bowen A, Chung CS, Cockburn J, Knapp P, Pollock A (2014) Rehabilitation for post-stroke cognitive impairment: an overview of recommendations arising from systematic reviews of current evidence. Clin Rehabil [Epub ahead of print]
Narasimhalu K, Wiryasaputra L, Sitoh YY, Kandiah N (2013) Post-stroke subjective cognitive impairment is associated with acute lacunar infarcts in the basal ganglia. Eur J Neurol 20:547–551
Park JH, Kim BJ, Bae HJ, Lee J, Lee J, Han MK, O KY, Park SH, Kang Y, Yu KH, Lee BC (2013) Impact of post-stroke cognitive impairment with no dementia on health-related quality of life. J Stroke 15:49–56
Pasi M, Poggesi A, Salvadori E, Pantoni L (2012) Post-stroke dementia and cognitive impairment. Front Neurol Neurosci 30:65–69
Acknowledgments
F. R. W. acknowledges project Grant support from the Australian National Health and Medical Research Council. M. N acknowledges the support from the University of Newcastle’s Brawn Fellowship, the Hunter Medical Research Institute (HMRI) and the Sten A. Olsson Foundation for Science and Culture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In honor of Michael Norenberg.
Madeleine J Patience and Ihssane Zouikr have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Patience, M.J., Zouikr, I., Jones, K. et al. Photothrombotic Stroke Induces Persistent Ipsilateral and Contralateral Astrogliosis in Key Cognitive Control Nuclei. Neurochem Res 40, 362–371 (2015). https://doi.org/10.1007/s11064-014-1487-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1487-8