Abstract
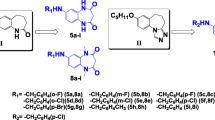
Epilepsy is one of the most common neurological diseases, with between 34 and 76 per 100,000 people developing epilepsy annually. Epilepsy therapy for the past 100+ years is based on the use of antiepileptic drugs (AEDs). Despite the availability of more than twenty old and new AEDs, approximately 30% of patients with epilepsy are not seizure-free with the existing medications. In addition, the clinical use of the existing AEDs is restricted by their side-effects, including the teratogenicity associated with valproic acid that restricts its use in women of child-bearing age. Thus, there is an unmet clinical need to develop new, effective AEDs. In the present study, a novel class of carbamates incorporating phenethyl or branched aliphatic chains with 6–9 carbons in their side-chain, and 4-benzenesulfonamide-carbamate moieties were synthesized and evaluated for their anticonvulsant activity, teratogenicity and carbonic anhydrase (CA) inhibition. Three of the ten newly synthesized carbamates showed anticonvulsant activity in the maximal-electroshock (MES) and 6 Hz tests in rodents. In mice, 3-methyl-2-propylpentyl(4-sulfamoylphenyl)carbamate(1), 3-methyl-pentan-2-yl-(4-sulfamoylphenyl)carbamate (9) and 3-methylpentyl, (4-sulfamoylphenyl)carbamate (10) had ED50 values of 136, 31 and 14 mg/kg (MES) and 74, 53, and 80 mg/kg (6 Hz), respectively. Compound (10) had rat-MES-ED50 = 13 mg/kg and ED50 of 59 mg/kg at the mouse-corneal-kindling test. These potent carbamates (1,9,10) induced neural tube defects only at doses markedly exceeding their anticonvuslnat-ED50 values. None of these compounds were potent inhibitors of CA IV, but inhibited CA isoforms I, II and VII. The anticonvulsant properties of these compounds and particularly compound 10 make them potential candidates for further evaluation and development as new AEDs.


Similar content being viewed by others
Abbreviations
- CMK:
-
Corneal kindled mouse
- CNS:
-
Central nervous system
- AED:
-
Antiepileptic drug
- SAR:
-
Structure–activity relationship
- MES:
-
Maximal electroshock seizure
- scMet:
-
Subcutaneous metrazol
- PI:
-
Protective index
- VPA:
-
Valproic acid
- SPD:
-
Sec-butylisopropylaceatmide
- THF:
-
Tetrahydrofuran
- DCM:
-
Dichlromethane
- NTD:
-
Neural tube defects
References
Brodie MS, Barry SE, Bamagous GA, Norrie JD, Kwan, Pattern P (2012) Treatment response to newly diagnosed epilepsy. Neurology 78:1548–1554
Fisher RS, van Emde BW, Blume W, Elger C, Genton P, Lee P, Engel J Jr (2005) Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46:470–472
Perucca E, French J, Bialer M (2007) Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol 6:793–804
Bialer M, White HS (2010) Key factors in the discovery and development of new antiepileptic drugs (AEDs). Nature Rev Drug Discov 9:68–83.
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS (2015) Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res 111:85–141
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS (2017) Progress report on new antiepileptic drugs: a summary of the Thirteenth Eilat Conference (EILAT XIII). Epilepsia. doi:10.1111/epi.13634
Bialer M, Yagen B (2007) Valproic acid: second generation. Neurother 4:130–137
Nau H, Loscher W (1986) Pharmacologic evaluation of various metabolites and analogs of valproic acid: teratogenic potencies in mice. Fundam Appl Toxicol 6:669–676
Bojic U, Elmazar MM, Hauck RS, Nau H (1996) Further branching of valproate-related carboxylic acids reduces the teratogenic activity, but not the anticonvulsant effect. Chem Res Toxicol 9:866–870
Liu MJ, Pollack GM (1994) Pharmacokinetics and pharmacodynamics of valproate analogues in rats. IV. Anticonvulsant action and neurotoxicity of octanoic acid, cyclohexanecarboxylic acid, and 1-methyl-1-cyclohexanecarboxylic acid. Epilepsia 35:234–243
Loscher W, Nau H (1985) Pharmacological evaluation of various metabolites and analogues of valproic acid. Anticonvulsant and toxic potencies in mice. Neuropharmacology 24:427–435
Bialer M (2012) Chemical properties of antiepileptic drugs (AEDs). Adv Drug Deliv Rev 64:887–895
Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, Hardege JD, Chen PE, Walker MC, Williams RS (2016) Seizure control by decanoic acid through direst AMPA receptor inhibition. Brain 139:431–443
Rogawski MA (2016) A fatty acid in MCT ketogenic diet for epilepsy treatment blocks AMPA receptors. Brain 139:306–309
Keane PE, Simiand J, Mendes E, Santucci V, Morre M (1983) The effects of analogues of valproic acid on seizures induced by pentylenetetrazol and GABA content in brain of mice. Neuropharmacology 22:875–879
Morre M, Keane PE, Vernieres JC, Simiand J, Roncucci R (1984) Valproate: recent findings and perspectives. Epilepsia 25(Suppl 1):5–9
Resor A SR, Resor LD, Woodbury DM, Kemp JW (1995) Acetazolamide. In: Levy RH, Mattson RH, Meldrum BS (eds) Antiepileptic Drugs, 4th edn, Raven Press, New York, pp 969–985.
Ganz AJ, Waser PG, Pfirrmann RW (1978) Development of new antiepileptics. V. Pharmacological activity of some derivatives of sulfanilamide (author’s transl). Arzneimittelforschung 28:1331–1334
Masereel B, Rolin S, Abbate F, Scozzafava A, Supuran CT (2002) Carbonic anhydrase inhibitors: anticonvulsant sulfonamides incorporating valproyl and other lipophilic moieties. J Med Chem 45:312–320
Tasso SM, Moon S, Bruno-Blanch LE, Estiu GL (2004) Characterization of the anticonvulsant profile of valpromide derivatives. Bioorg Med Chem 12:3857–3869
Hen N, Bialer M, Wlodarczyk B, Finnell RH, Yagen B (2010) Syntheses and evaluation of anticonvulsant profile and teratogenicity of novel amide derivatives of branched aliphatic carboxylic acids with 4-amino-benzensulfonamide. J Med Chem 53:4177–4186
Murray WJ, Kier LB (1977) Noncyclic anticonvulsants. In: Vida JA (ed) Medicinal chemistry. Academic Press, New York, pp 578–619
Ludwing BJ, Powell LS, Berger FM (1969) Carbamate derivatives related to meprobamate. J Med Chem 12:462–472
Pellock JM, Perhach JM, Sofia RD (2002) Felbamate. In: Levy RH, Mattson RH, Meldrum BS, m Perucca E (eds) Antiepileptic drugs, 5th edn, Lippincott, Wiliams & Wilkinss, Philadelphia, 301–318.
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS (2009) Progress report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX). Epilepsy Res 83:1–43
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS (2010) Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X). Epilepsy Res 92:89–124
Karauss G, French J, Kamin J, Ilankumaran P (2016) Seizure freedom with YKP3089 as adjunctive therapy for refractory partial-onset seizures in double-llind placebo-controlled trials. Neurology 86(16) P2–019
Hen N, Bialer M, Yagen B (2012) Syntheses and evaluation of anticonvulsant activity of novel branched alkyl carbamates. J Med Chem 55:2835–2845
Shekh-Ahmad T, Mawasi H, McDonough JH, Finnell RH, Wlodarczyk BJ, Bialer M (2014) Enantioselective pharmacodynamic and pharmacokinetic analysis of two active chiral CNS-active carbamate derivatives of valproic acid. Epilepsia 55:1944–1952
White HS, Woodhead JH, Wilcox KS, Stables JP, Kupferberg HJ, Wolf HH (2002) Discovery and preclinical development. In: Levy RH, Mattson RH, Meldrum BS, Perucca E (eds), Antiepileptic drugs, 5th edn, Lippincott Williams & Wilkins, New York, pp 36–48.
Smith M, Wilcox KS, White HS (2007) Discovery of antiepileptic drugs. Neurother 4:12–17
Finnell RH, Bennett GD, Karras SB, Mohl VK (1988) Common hierarchies of susceptibility to the induction of neural tube defects by valproic acid and its 4-propyl-4-pentenoic acid metabolite. Teratology 38:313–320
Wlodarczyk BC, Craig JC, Bennett GD, Calvin JA, Finnell RH (1996) Valproic acid–induced changes in gene expression during neurulation in a mouse model. Teratology 45:284–297
Khalifah RG (1971) The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 246:2561–2573
Casini A, Antel J, Abbate F, Scozzafava A, David S, Waldeck H, Schafer S, Supuran CT (2003) Carbonic anhydrase inhibitors: SAR and X-ray crystallographic study for the interaction of sugar sulfamates/sulfamides with isozymes I, II and IV. Bioorg. Med. Chem. Lett. 13:841–845
De Simone G, Di Fiore A, Menchise V, Pedone C, Antel J, Casini A, Scozzafava A, Wurl M, Supuran CT (2005) Carbonic anhydrase inhibitors. Zonisamide is an effective inhibitor of the cytosolic isozyme II and mitochondrial isozyme V: solution and X-ray crystallographic studies. Bioorg Med Chem Lett 15:2315–2320
Temperini C, Innocenti A, Scozzafava A, Parkkila S, Supuran CT (2010) The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example. J Med Chem 53:850–854
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev Drug Discov 7:168–181
Jezequel SG (1992) General nervous system penetration of drugs: importance of phisicochemical properties, Taylor & Francis London, pp 141–178.
White HS, Alex AB, Pollock A, Hen N, Shekh-Ahmed T, Wilcox KS, McDonough JH, Stables JP, Kaufmann D, Yagen B, Bialer M (2012) A new derivative of valproic acid amide possesses a broad-spectrum antiseizure profile and unique activity against status epilepticus and organophosphate neuronal damage. Epilepsia 53:134–146
Palaty J, Abbott FS (1995) Starcuture activity relestions of unsaturated nalpoogs use of valrproic acid. J Med Chem 38:3398–3406
Elamzar MMA, Hauck RS, Nau H (1993) Anticonvuslnat and neurotoxic activities of twelve anlaogues of valproic acid. J Pharm Sci 82:1255–1258
Halmi P, Parkkila S, Honkaniemi J (2006) Expression of carbonic anhydrases II, IV, VII, VIII and XII in rat brain after kainic acid induced status epilepticus. Neurochem Int 48:24–30
Supuran CT (2015) Acetazolamide for the treatment of idiopathic intracranial hypertension. Expert Rev Neurother 15:851–856
Thiry A, Dognè JM, Masereel B, Supuran CT (2007) Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem 7:855–864
Acknowledgements
This work is abstracted from the Ph.D. thesis of David Bibi in partial fulfillment of the Ph.D. degree requirements for The Hebrew University of Jerusalem. The authors thank Drs. John Keane and Shamsi Raeissi, of the NIH-NINDS Epilepsy Therapy Screening Program (ETSP) for testing the compounds in the ETSP and Dvora Izgelov and Bella Shusterman from the Hebrew University for their skillfully assistance with the HPLC and in the syntheses, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bibi, D., Mawasi, H., Nocentini, A. et al. Design and Comparative Evaluation of the Anticonvulsant Profile, Carbonic-Anhydrate Inhibition and Teratogenicity of Novel Carbamate Derivatives of Branched Aliphatic Carboxylic Acids with 4-Aminobenzensulfonamide. Neurochem Res 42, 1972–1982 (2017). https://doi.org/10.1007/s11064-017-2216-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2216-x