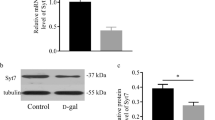
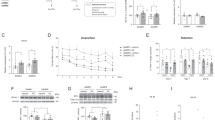
Abstract
As the elderly population rapidly increases worldwide, the onset of cognitive dysfunction is expected to increase. Although neuronal plasticity, neurogenesis, and mitochondrial dysfunction have been reported to be involved in cognitive function, the detailed mechanism of cognitive impairment accompanied by aging is poorly understood as there are many confounding factors associated with aging. Therefore, effective treatments for aging have not yet been developed, and the establishment of therapeutic strategies has not progressed accordingly. We have previously found a decline of cognitive function in the developmental stage in mice who lack the expression of Shati/Nat8l, an N-acetyl transferase However, the contribution of Shati/Nat8l to cognitive impairment in aged mice has not yet been investigated. In this study, we aimed to investigate the role of Shati/Nat8l in cognitive function during aging. We observed a reduction in Shati/Nat8l mRNA expression in the dorsal hippocampus of mice as a result of their aging. Moreover, the cognitive dysfunction observed in aged mice was reversed by Shati/Nat8l overexpression in the dorsal hippocampus. Shati/Nat8l overexpression in the dorsal hippocampus of mice did not alter the expression of neurotrophic factors or mitochondrial function-related genes, including Bdnf or Pgc-1α, which are suggested to be downstream genes of Shati/Nat8l. Decreased N-acetyl aspartate (NAA) in aged mice was upregulated by Shati/Nat8l overexpression, suggesting that the Shati/Nat8l-NAA pathway determines cognitive function with aging. Taken together, Shati/Nat8l and NAA in the dorsal hippocampus may be novel targets for the treatment of cognitive impairment.





Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Ni Y, Yang X, Zheng L, Wang Z, Wu L, Jiang J, Yang T, Ma L, Fu Z (2019) Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol Nutr Food Res 63:e1900603. https://doi.org/10.1002/mnfr.201900603
Riaz M, Vangberg TR, Vasylenko O, Castro-Chavira S, Gorecka MM, Waterloo K, Rodríguez-Aranda C (2021) What does hand motor function tell us about our aging brain in association with WMH? Aging Clin Exp Res 33:1577–1584. https://doi.org/10.1007/s40520-020-01683-0
Anderson ND, Craik FI (2017) 50 years of cognitive aging theory. J Gerontol B Psychol Sci Soc Sci 72:1–6. https://doi.org/10.1093/geronb/gbw108
Sengoku R (2020) Aging and Alzheimer’s disease pathology. Neuropathology 40:22–29. https://doi.org/10.1111/neup.12626
Chauhan A, Chauhan V (2020) Beneficial effects of walnuts on cognition and brain health. Nutrients 12:550. https://doi.org/10.3390/nu12020550
Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N (2017) Dementia prevention, intervention, and care. Lancet 390:2673–2734. https://doi.org/10.1016/S0140-6736(17)31363-6
Russell JK, Jones CK, Newhouse PA (2019) The role of estrogen in brain and cognitive aging. Neurotherapeutics 16:649–665. https://doi.org/10.1007/s13311-019-00766-9
Zahodne LB, Zajacova A (2020) Education and cognitive aging: an introduction to the special section. J Gerontol B Psychol Sci Soc Sci 75:e78–e80. https://doi.org/10.1093/geronb/gbaa091
Bartsch T, Wulff P (2015) The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience 309:1–16. https://doi.org/10.1016/j.neuroscience.2015.07.084
Bettio LEB, Rajendran L, Gil-Mohapel J (2017) The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev 79:66–86. https://doi.org/10.1016/j.neubiorev.2017.04.030
Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ (2018) Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22:589-599.e5. https://doi.org/10.1016/j.stem.2018.03.015
Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR (1991) Cerebral structure on MRI, part I: localization of age-related changes. Biol Psychiatry 29:55–67. https://doi.org/10.1016/0006-3223(91)90210-d
Golomb J, de Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH (1993) Hippocampal atrophy in normal aging. An association with recent memory impairment. Arch Neurol 50:967–973. https://doi.org/10.1001/archneur.1993.00540090066012
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, Rocktäschel P, Croteau DL, Akbari M, Greig NH, Fladby T, Nilsen H, Cader MZ, Mattson MP, Tavernarakis N, Bohr VA (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22:401–412. https://doi.org/10.1038/s41593-018-0332-9
Foster TC (2006) Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs 20:153–166. https://doi.org/10.2165/00023210-200620020-00006
Poulose SM, Miller MG, Scott T, Shukitt-Hale B (2017) Nutritional factors affecting adult neurogenesis and cognitive function. Adv Nutr 8:804–811. https://doi.org/10.3945/an.117.016261
Niwa M, Nitta A, Mizoguchi H, Ito Y, Noda Y, Nagai T, Nabeshima T (2007) A novel molecule “shati” is involved in methamphetamine-induced hyperlocomotion, sensitization, and conditioned place preference. J Neurosci 27:7604–7615. https://doi.org/10.1523/JNEUROSCI.1575-07.2007
Haddar M, Uno K, Azuma K, Muramatsu SI, Nitta A (2020) Inhibitory effects of Shati/Nat8l overexpression in the medial prefrontal cortex on methamphetamine-induced conditioned place preference in mice. Addict Biol 25:e12749. https://doi.org/10.1111/adb.12749
Miyanishi H, Uno K, Iwata M, Kikuchi Y, Yamamori H, Yasuda Y, Ohi K, Hashimoto R, Hattori K, Yoshida S, Goto YI, Sumiyoshi T, Nitta A (2020) Investigating DNA methylation of SHATI/NAT8L promoter sites in blood of unmedicated patients with major depressive disorder. Biol Pharm Bull 43:1067–1072. https://doi.org/10.1248/bpb.b19-01099
Miyanishi H, Nitta A (2021) A role of BDNF in the depression pathogenesis and a potential target as antidepressant: the modulator of stress sensitivity “Shati/Nat8l-BDNF system” in the dorsal striatum. Pharmaceuticals (Basel) 14:889. https://doi.org/10.3390/ph14090889
Nitta A, Noike H, Sumi K, Miyanishi H, Tanaka T, Takaoka K, Nagakura M, Iegaki N, Kaji JI, Miyamoto Y, Muramatsu SI, Uno K (2018) Shati/Nat8l and N-acetylaspartate (NAA) have important roles in regulating nicotinic acetylcholine receptors in neuronal and psychiatric diseases in animal models and humans. In: Akaike A, Shimohama S, Misu Y (eds) Nicotinic acetylcholine receptor signaling in neuroprotection. Springer, Singapore, pp 89–111
Sumi K, Uno K, Noike H, Tomohiro T, Hatanaka Y, Furukawa-Hibi Y, Nabeshima T, Miyamoto Y, Nitta A (2017) Behavioral impairment in SHATI/NAT8L knockout mice via dysfunction of myelination development. Sci Rep 7:16872. https://doi.org/10.1038/s41598-017-17151-1
Iida A, Takino N, Miyauchi H, Shimazaki K, Muramatsu S (2013) Systemic delivery of tyrosine-mutant AAV vectors results in robust transduction of neurons in adult mice. BioMed Res Int 2013:974819. https://doi.org/10.1155/2013/974819
Krzyzosiak A, Szyszka-Niagolov M, Wietrzych M, Gobaille S, Muramatsu S, Krezel W (2010) Retinoid X receptor gamma control of affective behaviors involves dopaminergic signaling in mice. Neuron 66:908–920. https://doi.org/10.1016/j.neuron.2010.05.004
Paxinos G, Franklin KBJ (2008) The mouse brain in stereotaxic coordinates: Compact, 3rd edn. Elsevier, Amsterdam
Uno K, Miyanishi H, Sodeyama K, Fujiwara T, Miyazaki T, Muramatsu SI, Nitta A (2019) Vulnerability to depressive behavior induced by overexpression of striatal Shati/Nat8l via the serotonergic neuronal pathway in mice. Behav Brain Res 376:112227. https://doi.org/10.1016/j.bbr.2019.112227
Miyamoto Y, Iegaki N, Fu K, Ishikawa Y, Sumi K, Azuma S, Uno K, Muramatsu SI, Nitta A (2017) Striatal N-acetylaspartate synthetase Shati/Nat8l regulates depression-like behaviors via mGluR3-mediated serotonergic suppression in mice. Int J Neuropsychopharmacol 20:1027–1035. https://doi.org/10.1093/ijnp/pyx078
Fu K, Miyamoto Y, Sumi K, Saika E, Muramatsu SI, Uno K, Nitta A (2017) Overexpression of transmembrane protein 168 in the mouse nucleus accumbens induces anxiety and sensorimotor gating deficit. PLoS ONE 12:e0189006. https://doi.org/10.1371/journal.pone.0189006
Miyamoto Y, Ishikawa Y, Iegaki N, Sumi K, Fu K, Sato K, Furukawa-Hibi Y, Muramatsu S, Nabeshima T, Uno K, Nitta A (2014) Overexpression of Shati/Nat8l, an N-acetyltransferase, in the nucleus accumbens attenuates the response to methamphetamine via activation of group II mGluRs in mice. Int J Neuropsychopharmacol 17:1283–1294. https://doi.org/10.1017/S146114571400011X
Haddar M, Azuma K, Izuo N, Kyosuke U, Asano T, Muramatsu SI, Nitta A (2021) Impairment of cognitive function induced by Shati/Nat8l overexpression in the prefrontal cortex of mice. Behav Brain Res 397:112938. https://doi.org/10.1016/j.bbr.2020.112938
Miyanishi H, Muramatsu SI, Nitta A (2021) Striatal Shati/Nat8l-BDNF pathways determine the sensitivity to social defeat stress in mice through epigenetic regulation. Neuropsychopharmacology 46:1594–1605. https://doi.org/10.1038/s41386-021-01033-2
Bolognin S, Buffelli M, Puoliväli J, Iqbal K (2014) Rescue of cognitive-aging by administration of a neurogenic and/or neurotrophic compound. Neurobiol Aging 35:2134–2146. https://doi.org/10.1016/j.neurobiolaging.2014.02.017
Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI (2015) The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis 6:331–341. https://doi.org/10.14336/AD.2015.0825
Molinari C, Morsanuto V, Ruga S, Notte F, Farghali M, Galla R, Uberti F (2020) The role of BDNF on aging-modulation markers. Brain Sci 10:285. https://doi.org/10.3390/brainsci10050285
Patterson SL (2015) Immune dysregulation and cognitive vulnerability in the aging brain: interactions of microglia, IL-1beta, BDNF and synaptic plasticity. Neuropharmacology 96:11–18. https://doi.org/10.1016/j.neuropharm.2014.12.020
Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (2008) New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 59:201–220. https://doi.org/10.1016/j.brainresrev.2008.07.007
Pietrelli A, Matković L, Vacotto M, Lopez-Costa JJ, Basso N, Brusco A (2018) Aerobic exercise upregulates the BDNF-serotonin systems and improves the cognitive function in rats. Neurobiol Learn Mem 155:528–542. https://doi.org/10.1016/j.nlm.2018.05.007
Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T (2007) Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res 85:525–535. https://doi.org/10.1002/jnr.21139
Palomer E, Martín-Segura A, Baliyan S, Ahmed T, Balschun D, Venero C, Martin MG, Dotti CG (2016) Aging triggers a repressive chromatin state at Bdnf promoters in hippocampal neurons. Cell Rep 16:2889–2900. https://doi.org/10.1016/j.celrep.2016.08.028
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736. https://doi.org/10.1146/annurev.neuro.24.1.677
Schliebs R, Arendt T (2011) The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221:555–563. https://doi.org/10.1016/j.bbr.2010.11.058
Fernandez A, Meechan DW, Karpinski BA, Paronett EM, Bryan CA, Rutz HL, Radin EA, Lubin N, Bonner ER, Popratiloff A, Rothblat LA, Maynard TM, LaMantia AS (2019) Mitochondrial dysfunction leads to cortical under-connectivity and cognitive impairment. Neuron 102:1127–1142. https://doi.org/10.1016/j.neuron.2019.04.013
Netto MB, de Oliveira Junior AN, Goldim M, Mathias K, Fileti ME, da Rosa N, Laurentino AO, de Farias BX, Costa AB, Rezin GT, Fortunato JJ, Giustina AD, Barichello T, Dal-Pizzol F, Petronilho F (2018) Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav Immun 73:661–669. https://doi.org/10.1016/j.bbi.2018.07.016
Reutzel M, Grewal R, Dilberger B, Silaidos C, Joppe A, Eckert GP (2020) Cerebral mitochondrial function and cognitive performance during aging: a longitudinal study in NMRI mice. Oxid Med Cell Longev 2020:4060769. https://doi.org/10.1155/2020/4060769
Pessentheiner AR, Pelzmann HJ, Walenta E, Schweiger M, Groschner LN, Graier WF, Kolb D, Uno K, Miyazaki T, Nitta A, Rieder D, Prokesch A, Bogner-Strauss JG (2013) NAT8L (N-acetyltransferase 8-like) accelerates lipid turnover and increases energy expenditure in brown adipocytes. J Biol Chem 288:36040–36051. https://doi.org/10.1074/jbc.M113.491324
Gleyzer N, Vercauteren K, Scarpulla RC (2005) Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25:1354–1366. https://doi.org/10.1128/MCB.25.4.1354-1366.2005
Ariyannur PS, Moffett JR, Manickam P, Pattabiraman N, Arun P, Nitta A, Nabeshima T, Madhavarao CN, Namboodiri AM (2010) Methamphetamine-induced neuronal protein NAT8L is the NAA biosynthetic enzyme: implications for specialized acetyl coenzyme A metabolism in the CNS. Brain Res 1335:1–13. https://doi.org/10.1016/j.brainres.2010.04.008
Neale JH, Olszewski RT, Zuo D, Janczura KJ, Profaci CP, Lavin KM, Madore JC, Bzdega T (2011) Advances in understanding the peptide neurotransmitter NAAG and appearance of a new member of the NAAG neuropeptide family. J Neurochem 118:490–498. https://doi.org/10.1111/j.1471-4159.2011.07338.x
Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP (2005) Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 384:23–28. https://doi.org/10.1016/j.neulet.2005.04.035
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. https://doi.org/10.1136/jnnp.20.1.11
Smith TD, Calhoun ME, Rapp PR (1999) Circuit and morphological specificity of synaptic change in the aged hippocampal formation. Neurobiol Aging 20:357–358. https://doi.org/10.1016/s0197-4580(99)00073-1
Dees RL, Kesner RP (2013) The role of the dorsal dentate gyrus in object and object-context recognition. Neurobiol Learn Mem 106:112–117. https://doi.org/10.1016/j.nlm.2013.07.013
Tuscher JJ, Taxier LR, Fortress AM, Frick KM (2018) Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol Learn Mem 156:103–116. https://doi.org/10.1016/j.nlm.2018.11.002
Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Initiative ADN (2014) What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog Neurobiol 117:20–40. https://doi.org/10.1016/j.pneurobio.2014.02.004
Fjell AM, Walhovd KB (2010) Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci 21:187–221. https://doi.org/10.1515/revneuro.2010.21.3.187
Malykhin NV, Bouchard TP, Camicioli R, Coupland NJ (2008) Aging hippocampus and amygdala. NeuroReport 19:543–547. https://doi.org/10.1097/WNR.0b013e3282f8b18c
Reichel JM, Bedenk BT, Czisch M, Wotjak CT (2017) Age-related cognitive decline coincides with accelerated volume loss of the dorsal but not ventral hippocampus in mice. Hippocampus 27:28–35. https://doi.org/10.1002/hipo.22668
Uno K, Miyazaki T, Sodeyama K, Miyamoto Y, Nitta A (2017) Methamphetamine induces Shati/Nat8L expression in the mouse nucleus accumbens via CREB- and dopamine D1 receptor-dependent mechanism. PLoS ONE 12:e0174196. https://doi.org/10.1371/journal.pone.0174196
Brightwell JJ, Gallagher M, Colombo PJ (2004) Hippocampal CREB1 but not CREB2 is decreased in aged rats with spatial memory impairments. Neurobiol Learn Mem 81:19–26. https://doi.org/10.1016/j.nlm.2003.08.001
Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A (2001) Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci 21:4066–4073. https://doi.org/10.1523/JNEUROSCI.21-11-04066.2001
Yu XW, Curlik DM, Oh MM, Yin JC, Disterhoft JF (2017) CREB overexpression in dorsal CA1 ameliorates long-term memory deficits in aged rats. eLife 6:e19358. https://doi.org/10.7554/eLife.19358
Heldt SA, Stanek L, Chhatwal JP, Ressler KJ (2007) Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12:656–670. https://doi.org/10.1038/sj.mp.4001957
Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD (2010) Histone methylation regulates memory formation. J Neurosci 30:3589–3599. https://doi.org/10.1523/JNEUROSCI.3732-09.2010
Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA (2015) DNA methylation of BDNF as a biomarker of early-life adversity. Proc Natl Acad Sci USA 112:6807–6813. https://doi.org/10.1073/pnas.1408355111
Jang JY, Blum A, Liu J, Finkel T (2018) The role of mitochondria in aging. J Clin Invest 128:3662–3670. https://doi.org/10.1172/JCI120842
Sumi K, Uno K, Matsumura S, Miyamoto Y, Furukawa-Hibi Y, Muramatsu S, Nabeshima T, Nitta A (2015) Induction of neuronal axon outgrowth by Shati/Nat8l by energy metabolism in mice cultured neurons. NeuroReport 26:740–746. https://doi.org/10.1097/WNR.0000000000000416
Bzdega T, Turi T, Wroblewska B, She D, Chung HS, Kim H, Neale JH (1997) Molecular cloning of a peptidase against N-acetylaspartylglutamate from a rat hippocampal cDNA library. J Neurochem 69:2270–2277. https://doi.org/10.1046/j.1471-4159.1997.69062270.x
Riederer F, Bittsanský M, Lehner-Baumgartner E, Baumgartner C, Mlynárik V, Gruber S, Moser E, Kaya M, Serles W (2007) Decrease of NAA with aging outside the seizure focus in mesial temporal lobe epilepsy–a proton-MRS study at 3 tesla. Brain Res 1179:131–139. https://doi.org/10.1016/j.brainres.2007.06.063
Chapman TW, Hill RA (2020) Myelin plasticity in adulthood and aging. Neurosci Lett 715:134645. https://doi.org/10.1016/j.neulet.2019.134645
Xin W, Chan JR (2020) Myelin plasticity: sculpting circuits in learning and memory. Nat Rev Neurosci 21:682–694. https://doi.org/10.1038/s41583-020-00379-8
Arshad M, Stanley JA, Raz N (2016) Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. Neuroimage 143:26–39. https://doi.org/10.1016/j.neuroimage.2016.08.047
Sullivan EV, Pfefferbaum A (2006) Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30:749–761. https://doi.org/10.1016/j.neubiorev.2006.06.002
Ahn JH, Lee TK, Park JH, Cho JH, Kim IH, Lee JC, Hong S, Jeon YH, Kang IJ, Lee YJ, Won MH, Lee CH (2017) Age-dependent differences in myelin basic protein expression in the hippocampus of young, adult and aged gerbils. Lab Anim Res 33:237–243. https://doi.org/10.5625/lar.2017.33.3.237
Namboodiri AM, Peethambaran A, Mathew R, Sambhu PA, Hershfield J, Moffett JR, Madhavarao CN (2006) Canavan disease and the role of N-acetylaspartate in myelin synthesis. Mol Cell Endocrinol 252:216–223. https://doi.org/10.1016/j.mce.2006.03.016
Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, Yuan J (2010) Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci USA 107:14164–14169. https://doi.org/10.1073/pnas.1009485107
Li P, Ma Y, Yu C, Wu S, Wang K, Yi H, Liang W (2021) Autophagy and aging: roles in skeletal muscle, eye, brain and hepatic tissue. Front Cell Dev Biol 9:752962. https://doi.org/10.3389/fcell.2021.752962
Yu Y, Feng L, Li J, Lan X, A L, Lv X, Zhang M, Chen L, (2017) The alteration of autophagy and apoptosis in the hippocampus of rats with natural aging-dependent cognitive deficits. Behav Brain Res 334:155–162. https://doi.org/10.1016/j.bbr.2017.07.003
Glatigny M, Moriceau S, Rivagorda M, Ramos-Brossier M, Nascimbeni AC, Lante F, Shanley MR, Boudarene N, Rousseaud A, Friedman AK, Settembre C, Kuperwasser N, Friedlander G, Buisson A, Morel E, Codogno P, Oury F (2019) Autophagy is required for memory formation and reverses age-related memory decline. Curr Biol 29:435–448. https://doi.org/10.1016/j.cub.2018.12.021
Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A (2011) TFEB links autophagy to lysosomal biogenesis. Science 332:1429–1433. https://doi.org/10.1126/science.1204592
Huber K, Hofer DC, Trefely S, Pelzmann HJ, Madreiter-Sokolowski C, Duta-Mare M, Schlager S, Trausinger G, Stryeck S, Graier WF, Kolb D, Magnes C, Snyder NW, Prokesch A, Kratky D, Madl T, Wellen KE, Bogner-Strauss JG (2019) N-acetylaspartate pathway is nutrient responsive and coordinates lipid and energy metabolism in brown adipocytes. Biochim Biophys Acta Mol Cell Res 1866:337–348. https://doi.org/10.1016/j.bbamcr.2018.08.017
Acknowledgements
We thank Naomi Takino and Mika Ito for technical assistance in producing the Shati/Nat8l vectors.
Funding
This research was supported by a Grant-in-Aid for Scientific Research (KAKENHI) (B) [JSPS KAKENHI Grant Number, 26293213, JP21H02632] (SM, AN), Kobayashi Foundation (AN), AdAMS (Ac210045, AN) and Smoking Research Foundation Grant for Biomedical Research and Foundation (AN). HM was supported by a Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan. The funders played no role in the study design, data collection or analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Hajime Miyanishi, Ayumu Kitazawa and Atsumi Nitta conceived and designed the study. Material preparation, data collection, and analysis were performed by Hajime Miyanishi, Ayumu Kitazawa, Naotaka Izuo, and Atsumi Nitta. AAV vectors were provided by Shin-ichi Muramatsu. The first draft of the manuscript was written by Hajime Miyanishi and it was confirmed by Atsumi Nitta. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
SM has equity in Gene Therapy Research Institution, Co., Ltd., which commercializes the use of AAV vectors for gene therapy applications. To the extent that the work in this manuscript increases the value of these commercial holdings, SM has conflicts of interest. The other authors have no relevant financial or nonfinancial interests to disclose.
Ethical Approval
All experimental procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and they were approved by the Committee for Animal Experiments at the University of Toyama (2021PHA-16, 20).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miyanishi, H., Kitazawa, A., Izuo, N. et al. N-Acetyl Transferase, Shati/Nat8l, in the Dorsal Hippocampus Suppresses Aging-induced Impairment of Cognitive Function in Mice. Neurochem Res 47, 2703–2714 (2022). https://doi.org/10.1007/s11064-022-03594-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03594-0