Abstract
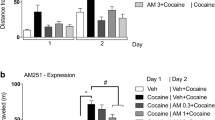
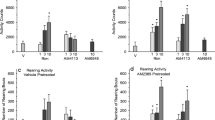
The number of cannabis users is increasing in the world. However, the mechanisms involved in the psychiatric effects and addiction formation remain unclear. Medical treatments against cannabis addiction have not yet been established. Δ9-Tetrahydrocannabinol (THC), the main active substance in cannabis, binds and affects cannabinoid type 1 receptors (CB1R) in the brain. The mice were intraperitoneally (i.p.) administered arachidonylcyclopropylamide (ACPA), a CB1R-selective agonist, and then two behavioral experiments on anxiety and addiction were performed. Administration of ACPA caused anxiolytic-like behavior in the elevated plus maze test. In addition, ACPA increased place preference in a conditioned place preference (CPP) test. The basolateral amygdala (BLA), which is the focus of this study, is involved in anxiety-like behavior and reward and is reported to express high levels of CB1R. We aimed to reveal the role of CB1R in BLA for ACPA-induced behavior. AM251, a CB1R selective antagonist, was administered intra-BLA before i.p. administration of ACPA. Intra-BLA administration of AM251 inhibited ACPA-induced anxiolytic-like behavior and place preference. These results suggest that CB1R in the BLA contributes to behavior disorders caused by the acute or chronic use of cannabis.




Similar content being viewed by others
Data Availability
The data presented in this manuscript are available upon request from the corresponding authors on reasonable request.
References
Cohen K, Weizman A, Weinstein A (2019) Positive and negative effects of cannabis and cannabinoids on health. Clin Pharmacol Ther 105:1139–1147. https://doi.org/10.1002/cpt.1381
Whiting PF, Wolff RF, Deshpande S, Nisio MD, Duffy S, Hernandez AV, Keurentjes JC, Lang SL, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J (2015) Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 313:2456–2473. https://doi.org/10.1001/jama.2015.6358
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D (2014) Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 82:1556–1563. https://doi.org/10.1212/WNL.0000000000000363
Sachs J, McGlade E, Yurgelun-Todd D (2015) Safety and toxicology of cannabinoids. Neurotherapeutics 12:735–746. https://doi.org/10.1007/s13311-015-0380-8
Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G (2002) Self-reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ 325:1199. https://doi.org/10.1136/bmj.325.7374.1199
Bally N, Zullino D, Aubry J (2014) Cannabis use and first manic episode. J Affect Disord 165:103–108. https://doi.org/10.1016/j.jad.2014.04.038
Gibbs M, Winsper C, Marwaha S, Gilbert E, Broome M, Singh SP (2015) Cannabis use and mania symptoms: a systematic review and meta-analysis. J Affect Disord 171:39–47. https://doi.org/10.1016/j.jad.2014.09.016
Zehra A, Burns J, Liu CK, Manza P, Wiers CE, Volkow ND, Wang G (2018) Cannabis addiction and the brain: a review. J Neuroimmune Pharmacol 13:438–452. https://doi.org/10.1007/s11481-018-9782-9
Demuth DG, Molleman A (2006) Cannabinoid signalling. Life Sci 78:549–563. https://doi.org/10.1016/j.lfs.2005.05.055
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564. https://doi.org/10.1038/346561a0
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of peripheral receptor for cannabis. Nature 365:61–65. https://doi.org/10.1038/365061a0
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11:563–583. https://doi.org/10.1523/JNEUROSCI.11-02-00563.1991
Tsou K, Brown S, Sanudo-peña MC, Walker JM (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411. https://doi.org/10.1016/S0306-4522(97)00436-3
Lutz B (2020) Neurobiology of cannabinoid receptor signaling. Dialogues Clin Neurosci 22:207–222. https://doi.org/10.31887/DCNS.2020.22.3/blutz
Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF (1999) Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19:4544–4558. https://doi.org/10.1523/JNEUROSCI.19-11-04544.1999
Monory K, Massa F, Egertova M, Egertová M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Jason L, Rubenstein JL, Goebbels S, Nave K, During M, Klugmann M, Wölfel B, Dodt H, Lutz B (2006) The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51:455–466. https://doi.org/10.1016/j.neuron.2006.07.006
Marsicano G, Lutz B (1999) Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11:4213–4225. https://doi.org/10.1046/j.1460-9568.1999.00847.x
Katona I, Urbán GM, Wallace M, Wallace M, Ledent C, Jung K, Piomelli D, Mackie K, Freund TF (2006) Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci 26:5628–5637. https://doi.org/10.1523/JNEUROSCI.0309-06.2006
Baxter MG, Murray EA (2002) The amygdala and reward. Nat Rev Neurosci 3:563–573. https://doi.org/10.1038/nrn875
McDonald AJ (1998) Cortical pathways to the mammalian amygdala. Prog Neurobiol 55:257–332. https://doi.org/10.1016/S0301-0082(98)00003-3
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. https://doi.org/10.1146/annurev.neuro.23.1.155
Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH (2008) Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature 453:1253–1257. https://doi.org/10.1038/nature06963
Kobb GF, Volkoe ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. https://doi.org/10.1038/npp.2009.110
Filbey FM, Dunlop J, Myers US (2013) Neural effects of positive and negative incentives during marijuana withdrawal. PLoS ONE. https://doi.org/10.1371/journal.pone.0061470
Heitzeg MM, Cope LM, Martz ME, Hardee JE, Zucker RA (2015) Brain activation to negative stimuli mediates a relationship between adolescent marijuana use and later emotional functioning. Dev Cogn Neurosci 16:71–83. https://doi.org/10.1016/j.dcn.2015.09.003
Spechler PA, Orr CA, Chaarani B, Kan KJ, Mackey S, Morton A, Snowe MP, Hudson KE, Althoff RR, Higgins ST, Cattrell A, Flor H, Nees F, Banaschewski T, Bokde ALW, Whelan R, Büchel C, Bromberg U, Conrod P, Frouin V, Papadopoulos D, Gallinat J, Heinz A, Walter H, Ittermann B, Gowland P, Paus T, Poustka L, Martinot JL, Artiges E, Smolka MN, Schumann G, IMAGEN Consortium (2015) Cannabis use in early adolescence: evidence of amygdala hypersensitivity to signals of threat. Dev Cogn Neurosci 16:63–70. https://doi.org/10.1016/j.dcn.2015.08.007
Wassum KM, Izuquierdo A (2015) The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev 57:271–283. https://doi.org/10.1016/j.neubiorev.2015.08.017
Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517:284–292. https://doi.org/10.1038/nature14188
Babaev O, Chatain CP, Krueger-burg D (2018) Inhibition in the amygdala anxiety circuitry. Exp Mol Med 50:18. https://doi.org/10.1038/s12276-018-0063-8
Katona I, Rancz EA, Acsády L, Ledent C, Mackie K, Hájos N, Freund T (2001) Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci 21(23):9506–9518. https://doi.org/10.1523/JNEUROSCI.21-23-09506.2001
Kamprath K, Romo-Parra H, Häring M, Gaburro S, Doengi M, Lutz B, Pape HC (2011) Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology 36:652–663. https://doi.org/10.1038/npp.2010.196
Lange MD, Daldrup T, Remmers F, Szkudlarek HJ, Lesting J, Guggenhuber S, Ruehle S, Jüngling K, Seidenbecher T, Lutz B, Pape HC (2017) Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat. Mol Psychiatry 22:1422–1430. https://doi.org/10.1038/mp.2016.156
Pertwee RG (2006) The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes. https://doi.org/10.1038/sj.ijo.0803272
Nasehi M, Sharaf-Dolgari E, Embrahimi-Ghiri M, Zarrindast M (2015) The hippocampal NMDA receptors may be involved in acquisition, but not expression of ACPA-induced place preference. Progr Neuro-Psychopharmacol Biol Psychiatry 63:83–90. https://doi.org/10.1016/j.pnpbp.2015.06.004
Ikeda H, Ikegami M, Kai M, Kamei J (2015) Cannabinoid functions in the amygdala contribute to conditioned fear memory in streptozotocin-induced diabetic mice: interaction with glutamatergic functions. Exp Neurol 269:233–241. https://doi.org/10.1016/j.expneurol.2015.04.012
Miyamoto Y, Noda Y, Komori Y, Sugihara H, Furukawa H, Nabeshima T (2000) Involvement of nitric oxide in phencyclidine-induced place aversion and preference in mice. Behav Brain Res 116:187–196. https://doi.org/10.1016/S0166-4328(00)00274-6
Miyamoto Y, Iida A, Sato K, Muramatsu S, Nitta A (2015) Knockdown of dopamine D2 receptors in the nucleus accumbens core suppresses methamphetamine-induced behaviors and signal transduction in mice. Int J Neuropsychopharmacol. https://doi.org/10.1093/ijnp/pyu038
Fu K, Miyamoto Y, Sumi K, Saika E, Muramatsu S, Uno K, Nitta A (2017) Overexpression of transmembrane protein 168 in the mouse nucleus accumbens induces anxiety and sensorimotor gating deficit. PLoS ONE. https://doi.org/10.1371/journal.pone.0189006
Miyanishi H, Muramatsu S, Nitta A (2021) Striatal Shati/Nat8l–BDNF pathways determine the sensitivity to social defeat stress in mice through epigenetic regulation. Neuropsychopharmacology 46:1594–1605. https://doi.org/10.1038/s41386-021-01033-2
Paxinos G, Franklin K (2008) The mouse brain in stereotaxic coordinates: compact, 3rd edn. Elsevier, Amsterdam
Jordan C, Xi ZX (2019) Progress in brain cannabinoid CB2 receptor research: from genes to behavior. Neurosci Biobehav Rev 98:208–202. https://doi.org/10.1016/j.neubiorev.2018.12.026
Lepore M, Vorel SR, Lowinson J, Gardner EL (1995) Conditioned place preference induced by Δ9-tetrahydrocannbinol: comparison with cocaine, morphine, and food reward. Life Sci 56:2073–2080. https://doi.org/10.1016/0024-3205(95)00191-8
Kangarlu-Haghighi K, Oryan S, Nasehi M, Zarrindast M (2015) The effect of BLA GABAA receptor in anxiolytic-like effect and aversive memory deficit induced by ACPA. EXCLI J 14:613–626. https://doi.org/10.17179/excli2015-201
Chegini H, NasehiZarrindast M (2014) Differential role of the basolateral amygdala 5-HT3 and 5-HT4 serotonin receptors upon ACPA-induced anxiolytic-like behaviors and emotional memory deficit in mice. Behav Brain Res 261:114–126. https://doi.org/10.1016/j.bbr.2013.12.007
Onavi ES, Green MR, Martin BR (1990) Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther 253:1002–1009
Berrendero F, Maldonado R (2002) Involvement of the opioid system in the anxiolytic-like effects induced by Δ9-tetrahydrocannabinol. Psychopharmacology 163:111–117. https://doi.org/10.1007/s00213-002-1144-9
Rubino T, Sala M, Viganò D, Braida D, Castiglioni C, Lirmonta V, Guidali C, Realini N, Parolaro D (2007) Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Δ9-tetrahydrocannabinol in rats. Neuropsychopharmacology 32:2036–2045. https://doi.org/10.1038/sj.npp.1301330
Shel H, Terrett G, Greenwood LM, Kowalczyk M, Thomson H, Poudel G, Manning V, Lorenzetti V (2021) Patterns of brain function associated with cannabis cue-reactivity in regular cannabis users: a systematic review of fMRI studies. Psychopharmacology 238:2709–2728. https://doi.org/10.1007/s00213-021-05973-x
Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE (2011) Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 36:2086–2096. https://doi.org/10.1038/npp.2011.99
Meil WM, See RE (1997) Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res 87:139–148. https://doi.org/10.1016/S0166-4328(96)02270-X
Bissiere S, Humeau Y, Luthi A (2003) Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci 6:587–592. https://doi.org/10.1038/nn1058
Woodruff AR, Sah P (2007) Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. J Neurophysiol 98:2956–2961. https://doi.org/10.1152/jn.00739.2007
Zarrindast M, Ahmadi S, Haeri-Rohani A, Rezayof A, Jafari M, Jafari-Sabet M (2004) GABAA receptors in the basolateral amygdala are involved in mediating morphine reward. Brain res 1006:49–58. https://doi.org/10.1016/j.brainres.2003.12.048
Diehl GW, Wachtel JM, Paine TA (2013) Cue-induced conditioned activity does not incubate but is mediated by the basolateral amygdala. Pharmacol Biochem Behav 104:69–79. https://doi.org/10.1016/j.pbb.2013.01.003
Crombag HS, Bossert JM, Koya E, Shaham Y (2008) Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B 363:3233–3243. https://doi.org/10.1098/rstb.2008.0090
Vinklerova J, Novakova J, Sulcova A (2002) Inhibition of methamphetamine self-administration rats by cannabinoid receptor antagonist AM251. Psychopharmacology 16:139–143. https://doi.org/10.1177/026988110201600204
Schindler CW, Panlilio LV, Gilman ZJ, Vemuri VK, Makriyannis A, Goldberg SR (2010) Effects of cannabinoid receptor antagonists on maintenance and reinstatement of methamphetamine self-administration in rhesus monkeys. Eur J Pharmacol 633:44–49. https://doi.org/10.1016/j.ejphar.2010.02.005
Guiffrida A, Parsons LH, Kerr TM, de Fonseca R, Navarro M, Piomelli D (1999) Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2:358–363. https://doi.org/10.1038/7268
Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agro A, Bernardi G, Calabresi P, Maccarrone M (2004) A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology 29:1488–1497. https://doi.org/10.1038/sj.npp.1300458
Lutz B, Marsicano G, Maldonado R, Hillard CJ (2015) The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 16:705–718. https://doi.org/10.1038/nrn4036
Saugy M, Avois L, Saudan C, Robinson N, Giroud C, Mangin P, Dvorak J (2006) Cannabis and sport. Br J Sports Med 40:13–15. https://doi.org/10.1136/bjsm.2006.027607
Sharpe L, Sinclair J, Kramer A, de Manincor M, Sarris J (2020) Cannabis, a cause for anxiety? A critical appraisal of the anxiogenic and anxiolytic properties. J Transl Med. https://doi.org/10.1186/s12967-020-02518-2
McDonald AJ, Mascagni F (2001) Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience 107:641–652. https://doi.org/10.1016/S0306-4522(01)00380-3
Shindo S, Yoshioka N (2005) Polymorphisms of the cholecystokinin gene promoter region in suicide victims in Japan. Forensic Sci Int 150:85–90. https://doi.org/10.1016/j.forsciint.2004.10.001
Berna MJ, Tapia TA, Sancho V, Jensen RT (2007) Progress in developing cholecystokinin (CCK)/gastrin receptor ligands that have therapeutic potential. Curr Opin Pharmacol 7:583–592. https://doi.org/10.1016/j.coph.2007.09.011
Rotzinger S, Lovejoy DA, Tan LA (2010) Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 31:736–756. https://doi.org/10.1016/j.peptides.2009.12.015
Frankland PW, Josselyn SA, Bradwejn J, Vaccarino FJ, Yeomans JS (1997) Activation of amygdala cholecystokininB receptors potentiates the acoustic startle response in the rat. J Neurosci 17:1838–1847. https://doi.org/10.1523/JNEUROSCI.17-05-01838.1997
Boca CD, Lutz PE, Le Merrer J, Koebel P, Kieffer BL (2012) Cholecystokinin knock-down in the basolateral amygdala has anxiolytic and antidepressant-like effects in mice. Neuroscience 218:185–195. https://doi.org/10.1016/j.neuroscience.2012.05.022
Prager EM, Bergstrom HC, Wynn GH, Braga MF (2016) The basolateral amygdala GABAergic system in health and disease. J Neurosci Res. https://doi.org/10.1002/jnr.23690
Barbalho CA, Nunes-de-Souza RL, Canto-de-Souza A (2009) Similar anxiolytic-like effects following intra-amygdala infusions of benzodiazepine receptor agonist and antagonist: evidence for the release of an endogenous benzodiazepine inverse agonist in mice exposed to elevated plus-maze test. Brain Res 1267:65–76. https://doi.org/10.1016/j.brainres.2009.02.042
Varodayan FP, Bajo M, Soni N, Luu G, Madamba SG, Schweitzer P, Roberto M (2016) Chronic alcohol exposure disrupts CB1 regulation of GABAergic transmission in the rat basolateral amygdala. Addict Biol 22:766–778. https://doi.org/10.1111/adb.12369
Acknowledgements
We thank Prof Satoshi Morimoto, Kyusyu University, for supplying THC for pre-study.
Funding
This work was supported by the Grant-in-aid for Scientific Research (KAKENHI) (B) [JSPS KAKENHI JP21H02632], JP22J11998, JP16H06276 (AdAMS) from the Japan Society for the Promotion of Science, Kobayashi Foundation, and SRF Grant for Biomedical Research and Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of the study. Material preparation, data collection and analysis were performed by TT, TA, and SN. The first manuscript was written by TT and HM. AN revised the manuscript to the final version. All the authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Animal experimental protocols were by the animal Care and Use Committee of the University of Toyama (Approval Number: A2020PHA14) and conducted in accordance with the Institutional Animal Experiment Handling Rules of the University of Toyama. The number of animals used was carefully estimated and kept to the minimum necessary for meaningful interpretation of the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tokutake, T., Asano, T., Miyanishi, H. et al. Cannabinoid Type 1 Receptors in the Basolateral Amygdala Regulate ACPA-Induced Place Preference and Anxiolytic-Like Behaviors. Neurochem Res 47, 2899–2908 (2022). https://doi.org/10.1007/s11064-022-03708-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03708-8