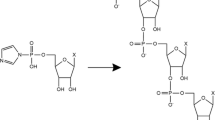
Abstract
Demonstration of the abiotic polymerization of ribonucleotides under conditions consistent with conditions that may have existed on the prebiotic Earth is an important goal in “RNA world” research. Recent reports of abiotic RNA polymerization with and without catalysis rely on techniques such as HPLC, gel electrophoresis, and MALDI-TOF MS to analyze the reaction products. It is essential to understand the limitations of these techniques in order to accurately interpret the results of these analyses. In particular, techniques that rely on mass for peak identification may not be able to distinguish between a single, linear RNA oligomer and stable aggregates of smaller linear and/or cyclic RNA molecules. In the case of MALDI-TOF MS, additional complications may arise from formation of salt adducts and MALDI matrix complexes. This is especially true for abiotic RNA polymerization reactions because the concentration of longer RNA chains can be quite low and RNA, as a polyelectrolyte, is highly susceptible to adduct formation and aggregation. Here we focus on MALDI-TOF MS analysis of abiotic polymerization products of imidazole-activated AMP in the presence and absence of montmorillonite clay as a catalyst. A low molecular weight oligonucleotide standard designed for use in MALDI-TOF MS and a 3′-5′ polyadenosine monophosphate reference standard were also run for comparison and calibration. Clay-catalyzed reaction products of activated GMP and UMP were also examined. The results illustrate the ambiguities associated with assignment of m/z values in MALDI mass spectra and the need for accurate calibration of mass spectra and careful sample preparation to minimize the formation of adducts and other complications arising from the MALDI process.
Similar content being viewed by others
Introduction
All life as we know it must be able to process chemicals from its environment to create its biological machinery and replicate the genetic material that instructs the organism how to create this machinery. Ribonucleic Acid (RNA) is an intriguing molecule in that it can serve as both a template for replication and as the machinery that conducts this replication (Guerrier-Takada et al. 1983; Kruger et al. 1982). Due to these properties, it is possible that life could arise from systems in which RNA served as both the genetic and metabolic material (Orgel 1986, Gesteland et al. 2006). The notion of organisms dominated by RNA-based biochemistry is popularly referred to as the “RNA world”.
A central goal in RNA world research is the abiotic synthesis of 3′-5′ RNA polymers from monomeric ribonucleic acids under conditions consistent with possible conditions on the early Earth. [Note that this assumes that there was an abiotic route to ribonucleotide synthesis either directly from smaller molecules (see for example the discussion in Sutherland 2010) or through evolution of a more readily synthesized precursor, or “proto-RNA” (Engelhart and Hud 2010), which is a key consideration but one that is outside the scope of the present work]. Researchers have proposed and explored various routes to abiotic RNA polymerization, some based on involvement of minerals or small molecules as catalysts or facilitators (Ferris 2005, 2006; Joshi et al. 2012; Hansma 2010; Hazen and Sverjensky 2010; Horowitz et al. 2010) and others based on polymerization of 3′-5′ cyclic ribonucleotide monomers without such assistance (Pino et al. 2008; Costanzo et al. 2009, 2011, 2012). Fundamental to these efforts is the need for analytical methods to characterize the products of these polymerization reactions. Traditionally, High Performance Liquid Chromatography (HPLC) (Ferris and Ertem 1993; Zagorevskii et al. 2006), and Gel Electrophoresis (GE) (Pino et al. 2008; Costanzo et al. 2009, 2012) have been used for this purpose, with Matrix Assisted Laser Desorption Ionization–Time-of-Flight Mass Spectrometry (MALDI-TOF MS) being recently explored as well (Zagorevskii et al. 2006; Costanzo et al. 2012). In one report, MALDI-TOF MS was able to detect RNA products as long as 30-mers from the montmorillonite-catalyzed polymerization of imidazole-activated monomers, while HPLC was only able to detect 12-mers in the same sample (Zagorevskii et al. 2006). This suggests that MALDI-TOF MS can extend the range of detection to much longer RNA products. This, combined with the mass information provided by MS, makes MALDI-TOF MS a promising addition to the RNA analysis toolbox.
While recognizing the strengths of MALDI-TOF MS, it is important to understand its limitations as well. In some cases, m/z peaks that are attributed to a single, linear RNA oligomer product might also be explained by non-covalent aggregation of shorter, linear and/or cyclic oligomers. These small oligomers may be formed as reaction products or could be present as minor contaminants in the initial reactants. Formation of oligomer aggregates, particularly in the case of the purines AMP and GMP that are prone to hydrogen-bond formation and π-π stacking interactions, are well known and have shown resistance to conventional denaturing procedures (Rich et al. 1961; Brahms et al. 1966; Davis 2004; Wang and Kool 1994). Such aggregates could form in solution or during the MALDI-TOF plume expansion and give rise to peaks at the predicted masses for individual, linear oligomerization products and be misidentified as such. Aggregation presents an alternative explanation for reported observations of abiotic RNA polymerization of 3′,5′cyclic ribonucleotides without a catalyst (Pino et al. 2008; Costanzo et al. 2009, 2011, 2012). The authors report that other laboratories have had difficulty reproducing their results (Costanzo et al. 2011). They attribute this irreproducibility to interference by sodium ions and stress the need to use the free acid form of the ribonucleotides; however, it is also possible that the observed mass spectral peaks correspond to aggregates of shorter cyclic and linear oligomers rather than to single, longer linear oligomers. For example, a linear 8-mer of G (2779.8 amu) has an equivalent mass of an aggregate formed from a linear 4-mer of G and a cyclic 4-mer of G (1398.9 amu + 1380.9 amu = 2779.8 amu), or other combinations of linear and cyclic oligomers (5 + 3, 6 + 2, etc.). It would be difficult to distinguish between these two possibilities using MALDI-TOF MS since both would yield the same m/z peaks. It is worth noting that gel electrophoresis also could be misleading since high molecular weight bands could be due to linear polymers or aggregates of smaller cyclic and linear oligomers. Since electrophoretic mobility would depend upon both m/z and shape, linear oligonucleotide standards would not provide definitive masses for the peaks. Thus, dependence on either or both of these techniques might lead to erroneous conclusions about the extent of polymerization.
MALDI-TOF MS has also been used to confirm the presence of linear polymerization products as long as 40-mers from montmorillonite-catalyzed reactions of imidazole-activated guanosine (ImpG) and adenosine monophosphate (ImpA) (Joshi et al. 2012; Zagorevskii et al. 2006). In these cases, peaks could be due instead to aggregates of low molecular weight cyclic oligomers with short linear oligomers and possibly even monomers, particularly because of the absence of peaks corresponding to odd-numbered oligomers in the mass spectra for the ImpG reaction products. In this case, aggregates would be expected due to the well known self-assembly of guanosine compounds to form intermolecular G-tetrad structures (Davis 2004) and only peaks at m/z corresponding to even-numbered oligomers would be observed. The adenosine oligomers may also form clusters leading to aggregation during MALDI-TOF MS analysis. Due to potentially identical m/z values, aggregation from both sets of these reaction products can make it difficult to judge the effectiveness of these reactions in forming longer-chain oligomers.
In order to examine these alternate explanations for MALDI-TOF mass spectra of RNA polymerization products, we performed MALDI-TOF MS analysis of abiotic polymerization products of imidazole-activated AMP, GMP, and UMP (ImpA, ImpG and ImpU) in the presence of montmorillonite clay. The emphasis is on ImpA, which was run in both the presence and absence of catalytic clay and for which the results were compared with MALDI-TOF mass spectra of a low molecular weight oligonucleotide standard (LMW standard) designed for use in MALDI-TOF MS, and a 3′-5′ polyadenosine monophosphate reference standard (polyA standard). The analyses were performed using different MALDI matrices, target surfaces, and sample pretreatment methods as these variables all can play critical roles in the MALDI-TOF MS results, for example, by promoting formation of adducts and complexes that might complicate interpretation of the mass spectra (Sarracino and Richert 1996; Distler and Allison 2001; Cassidy et al. 2011). These considerations are particularly important for abiotic RNA polymerization reactions because the concentration of longer RNA chains can be quite low and RNA is highly susceptible to adduct formation and aggregation (Pieles et al. 1993). MALDI-TOF MS analysis of polymerization reaction products of ImpG and ImpU provided further insight into ambiguities associated with interpretation of the MALDI mass spectra.
Materials and Methods
Materials
Fused silica plates were obtained from Valley Design (Shirley, MA, USA). 2,4,6-trihydroxyacetophenone (THAP), 5-methoxysalicylic acid (MSA), 3-hydroxypicolinic acid (HPA), adenosine-5′-monophosphate (AMP), disodium guanosine-5′-monophosphate (GMP), disodium uridine-5′-monophosphate (UMP), 50 % ammonium hydroxide, Dowex 50WX8 cation exchange resin, and the 3′-5′ polyadenosine monophosphate reference standard (polyA standard, mass values: 1173.8, 1487.0, 2105.4, 2722.8, 3341.2 Da) were purchased from Sigma (St. Louis, MO, USA). Montmorillonite in the form of volclay SPV-200 was a gift from The American Colloid Company (Arlington Heights, IL, USA). Low molecular weight oligonucleotide calibration standard (LMW standard) comprising 4-mer, 5-mer, 7-mer, 9-mer, and 11-mer oligonucleotides was purchased from Bruker Daltonics (Billerica, MA, USA). Ultrapure, 18 MΩ cm−1 deionized and autoclaved water was used for all sample preparations and experiments.
Oligomerization Procedure
Disodium GMP and UMP were converted to their acidic form using hydrogen saturated Dowex 50WX8 cation exchange resin. Monomeric acid-form AMP, GMP, and UMP were activated by adding imidazole to the 5′-phosphate, as described previously (Pfeffer et al. 2005). Solutions of AMP or ImpX, where X = A, G, or U, were prepared by dissolving the compound to a concentration of 15 mM in RNase-free water containing 0.075 M MgCl2 and 0.2 M NaCl. Homoionic Na-montmorillonite clay was prepared according to the procedure of Banin et al. (1985). 200 μL of the ImpX or AMP solution was added to 10 mg of the lyophilized Na-montmorillonite clay and vortexed to form a homogenous mixture. After 3 days of continuous agitation, the sample was centrifuged and the supernatant was collected and subjected to Millipore 0.6 μL C18 Zip Tip purification following the manufacturer’s guidelines.
MALDI-TOF MS Analysis
MALDI-TOF MS was performed using a Bruker Autoflex II instrument (Bruker Daltonics). The targets included an AnchorChip var/387 steel surface, 5 × 5 cm fused silica plates, and a MTP 384 massive stainless steel target that was modified in-house to accommodate the four silica plates. The AnchorChip and steel targets were also acquired from Bruker Daltonics. The MALDI matrices THAP, HPA, and MSA were utilized following published protocols using appropriate dopants (ammonium citrate for THAP and HPA, spermine for MSA) to reduce adduct formation (Sarracino and Richert 1996; Distler and Allison 2001; Castleberry et al. 2008). All analyses were performed in negative ion mode. The RNA polymerization products were analyzed in reflectron mode with the following settings: ion source 1, 19.00 kV; ion source 2, 16.85 kV; lens, 8.5 kV; reflector, 20.0 kV; system energy, 115.7 ± 5.0 μJ; pulsed ion ext., 80–400 ns. In all cases, relative laser power for desorption was 31 %–55 % for all targets. 400 laser shots were applied per location. The shots were summed and smoothed using the Savitsky-Golay polynomial regression algorithm with a width of 0.2 m/z. Baseline subtraction was used on all spectra. Prior to analysis, the mass spectrometer was calibrated using the low molecular oligonucleotide standards as external calibrants. Once external calibration was performed, the polymerization products from each reaction were used as internal calibration points as long as their m/z values were within ±0.5 m/z of their theoretical values.
HPLC Analysis
Phenomenex Phenex Nylon 0.20-micron syringe filters were used to remove particulates from the samples prior to analysis. Reverse-phase HPLC analysis was performed on a Hitachi L-6200A intelligent pump system equipped with a Hitachi L-4000 UV detector operating at 260 nm. The negatively charged products were separated on a Dionex DNA PacR-100 μm (4.0 × 250 mm) analytical anion exchange column from Dionex Corporation, Sunnyvale, CA, USA, using a gradient of 0 – 0.4 M NaClO4 with 2 mM Tris at pH 8.
Results and Discussion
Experimental Precautions and Optimization of RNA Detection
Due to the complex nature of the MALDI process, MALDI-TOF MS is particularly sensitive to experimental conditions such as the presence of salts and dust in the sample, the material used for the MALDI target, and the MALDI matrix used. These all can have dramatic consequences for MALDI-TOF MS analysis, especially of abiotic RNA polymerization products that typically occur at low yields from reactions performed in complex solutions containing salts and catalytic materials such as clays. In light of this sensitivity, our first step towards investigating the potential misattribution of mass spectral peaks was to examine the MALDI-TOF MS experiment itself.
Sample Pretreatment
Salts, dust, and other sample contaminants can lead to formation of adducts and complexes containing analyte, salt, and MALDI matrix molecules that will give rise to artifactual peaks in the mass spectrum. The addition of dopants (e.g., ammonium citrate or spermine) to the MALDI matrix has been shown to suppress formation of complexes and adducts (Zhu et al. 1996; Distler and Allison 2001). We found, however, that the dopants were insufficient to fully suppress formation of these unwanted species. It was necessary to take additional precautions to minimize effects of salts, dust, and other interfering sample contaminants. These precautions included using C18 zip tips to greatly reduce alkali cation concentrations and performing the crystallization of the sample with the MALDI matrix in a dust-free environment. The importance of these steps is shown in Fig. 1 for a solution of unactivated AMP using THAP matrix doped with ammonium citrate. This reaction mixture should not contain any polymerization products, yet analysis of the sample that had not been subjected to the pretreatment steps shows groups of peaks, each separated from the next by differences in the range of m/z 347–386 that correspond to AMP monomers and their salt adducts (Fig. 1a). This pattern of peaks appeared consistently in untreated samples for all of the MALDI matrices and surfaces used in this study (data not shown). The absence of these peaks in samples that were prepared using the above-mentioned precautions (Fig. 1b) indicates that these peaks are not due to polymerization products, but instead to nucleation of AMP and matrix on dust and salt in the sample, producing clusters and adducts that fall in the same range and exhibit similar spacing as the expected reaction products.
MALDI-TOF MS spectra of unactivated AMP without clay using THAP matrix doped with ammonium citrate on a steel AnchorChip surface without (a) and with (b) precautions including sample pretreatment using C18 zip tips and performing the crystallization of the sample with the MALDI matrix in a dust-free environment. [m/z values: [(AMP)2 + (2,4,6 THAP)2 + K+ - (H+)2] 1067.8, [(AMP)3 + (2,4,6 THAP)2 + K+ - (H+)2] 1415.1, [(AMP)4 + (2,4,6 THAP)2 + (K+)2 - (H+)3]] 1800.4. Additional sodium adducts can also be observed near these listed masses
This important result clearly demonstrates that matrix dopants alone are not sufficient to suppress formation of complexes and adducts of both analyte and matrix. Additional precautions are needed to prevent the appearance of artifactual peaks that could be mistakenly attributed to polymerization products. In this case, the artifactual peaks in Fig. 1a could lead one to erroneously conclude that AMP could spontaneously polymerize without activation and in the absence of a catalyst/facilitator.
MALDI Target
Although bulk metals such as gold, aluminum, or stainless steel are generally used as the target surface in MALDI-TOF MS, our group previously reported the enhancement of higher m/z peaks for certain polymers including RNA on fused silica compared to steel targets (Cassidy et al. 2011). In the present work we analyzed the LMW standard and the montmorillonite-catalyzed ImpA polymerization products on stainless steel, fused silica, and stainless steel AnchorChip targets. AnchorChip targets are stainless steel targets with 400 μm hydrophilic spots designed to provide uniform analyte distribution and aid in analytical reproducibility.
Results (Online Resource 1 Figures S1 and S2) show that the steel AnchorChip provides the best results for both the LMW standard and the polymerization products. These results were independent of instrumental settings used to improve peak resolution and intensity, such as the voltages of the ion source and lens, the pulsed ion extraction delay, and the gain on the reflectron. The steel AnchorChip surface, therefore, was used in the remainder of the experiments.
MALDI Matrix
It is well known that the quality of MALDI-TOF MS results is dependent on the chosen MALDI matrix, and so it is important to examine the effect of a given matrix on the overall signal intensity and promotion of artifactual peaks for a particular type of analyte. Toward this end, initial studies were conducted using the LMW standard with three different MALDI matrices previously shown to give strong analytical signals for RNA: HPA, MSA, and THAP (Sarracino and Richert 1996; Distler and Allison 2001; Castleberry et al. 2008). The LMW standard contains five generic oligonucleotides and should yield five readily observable peaks. Under our experimental conditions, the expected five peaks showed a strong signal using HPA and THAP, while the 11-mer peak was barely discernible using MSA (Fig. 2). This suggests that THAP and HPA are more suitable matrices than MSA for the oligomers at low concentrations.
The three matrices were then compared for analysis of the RNA polymerization products. The THAP matrix provided the cleanest spectra with the highest peak intensities and S/N, followed by HPA and then MSA. The mass spectra for m/z 1,000–3,500 and m/z 3,750–5,750 are shown in Figs. 3 and 4, respectively. The same trend was observed for ImpA without catalytic clay (Fig. 5). There is, however, an anomaly in the spectra in Fig. 5 in that peaks are observed in the higher m/z region (m/z >1,000), which suggests the presence of polyA oligomerization products up to the 10-mer (m/z 3,307). This is surprising since oligomers larger than 3-mers should not be observed for ImpA in the absence of the catalytic clay (Ferris 2005). HPLC analysis was then conducted on the contents of both solutions (ImpA reaction products with and without clay). The results (Fig. 6) show higher-order oligomers only in the sample containing clay, which indicates that the mass spectral peaks observed for ImpA in the absence of clay in Fig. 5 are more likely due to aggregates of smaller strands than to higher-order polyA oligomers. The latter cannot be ruled out, however, since longer oligomers, if present in the reaction without clay, would occur at very low concentrations that could be detectable by MALDI-TOF MS but not by HPLC.
MALDI-TOF MS spectra in low m/z range of montmorillonite-catalyzed ImpA reaction products [m/z values: 3-mer 1004.7, 4-mer 1333.9, 5-mer 1663.1, 6-mer 1992.3, 7-mer 2321.5, 8-mer 2650.8, 9-mer 2980.0, (9-mer +18) 2998.0, (10-mer + 18) 3327.2] analyzed on steel AnchorChip using (a) MSA with spermine, (b) HPA with ammonium citrate, and (c) THAP with ammonium citrate. Each increase of m/z 329 represents addition of an AMP subunit
MALDI-TOF MS spectra in high m/z range of Montmorillonite-catalyzed ImpA reaction products [m/z values: 12-mer + (18)1 (3985.6), 13-mer + (18)2 (4332.9), 14-mer + (18)2 (4662.1), 15-mer + (18)2 (4991.3), 16-mer + (18)2 (5320.5)] analyzed on steel AnchorChip using (a) MSA with spermine, (b) HPA with ammonium citrate, and (c) THAP with ammonium citrate
MALDI-TOF MS spectra of ImpA polymerization reaction products [m/z values: 3-mer (1004.7), 4-mer + (18)1 (1351.9), 5-mer + (18)1 (1681.1), 6-mer + (18)2 (2028.3), 7-mer + (18)2 (2357.5), 8-mer + (18)3 (2704.8), 9-mer + (18)3 (3034.0), 10-mer + (18)4 (3381.2)] performed in the absence of clay on steel AnchorChip using (a) MSA with spermine, (b) HPA with ammonium citrate, and (c) THAP with ammonium citrate
Comparison of the spectra in Figs. 4 and 5 with that of the polyA reference standard (Fig. 7a) provides insight into the reaction products. The m/z values for the polyA oligomerization products exhibit strong peaks that can be attributed to oligomers up to 7-mer, with weaker signals at the 8-mer and 9-mer positions. This is consistent with the HPLC analysis in Fig. 6. However, at higher m/z values, peaks corresponding to [oligomer + (18)n] become the primary species in this reaction mixture. Comparing this mixture with the polyA reference standard, it can be seen that these peaks do not correspond to polyA or dehydrated polyA products (Fig. 7b and c). One possibility is that these peaks arise from aggregates of shorter polyA strands, which are known to aggregate under acidic conditions (Rich et al. 1961; Brahms et al. 1966). Surprisingly, the [oligomer + (18)n] peaks are not evident for the polyA reference standard, implying that these aggregates are not simply due to clustering of linear polyA oligomers. Highly stable aggregates of cyclic and linear oligonucleotides have been shown to form in solution under acidic conditions (Wang and Kool 1994). Such aggregates would be more resistant to dissociation during MALDI-TOF MS analysis, leading to the [oligomer + (18)n] m/z peaks in our spectra. Since acidic conditions favor polyA aggregation, we next ran the polyA oligomerization reaction products using with a THAP matrix that had been titrated to pH 10 with ammonium hydroxide. The results show a marked decrease in the relative intensity for the [oligomer + (18)n] m/z peaks (Fig. 8), which is consistent with attribution of those peaks to polyA aggregation in the reaction performed under more acidic conditions.
MALDI-TOF MS spectra on steel AnchorChip using THAP matrix with ammonium citrate. a PolyA reference standard. Dehydration products for each oligomer can be observed at m/z (oligomer-18). b Montmorillonite-catalyzed polymerization products. Products up to the 7-mer are observed before the m/z (oligomer + 18) peaks become the dominant species. c The combined mixture of montmorillonite reaction products and polyA standard showing non-overlap of the peaks and clearly defined species from the individual reaction mixtures
MALDI-TOF MS spectra on steel AnchorChip using THAP matrix with ammonium citrate. a Montmorillonite-catalyzed ImpA polymerization products in standard THAP matrix with ammonium citrate. b Montmorillonite-catalyzed polymerization products in THAP matrix with ammonium citrate titrated to pH 10 with ammonium hydroxide showing increase in intensity for the oligomer peaks relative to the [oligomer + (18)n] species
To further investigate aggregate formation, clay-catalyzed ImpG and ImpU polymerization reactions were run under identical conditions as the ImpA reactions. The reaction products exhibited peaks consistent with oligomer formation (Fig. 9). The [oligomer + (18)n] peaks also were observed for the ImpG reaction (Fig. 9b), but not for the ImpU reaction (Fig. 9c). This result supports aggregate formation because RNA oligomers of adenosine and guanosine are known to aggregate (Rich et al. 1961; Brahms et al. 1966; Davis 2004) whereas uridine oligomers do not appreciably aggregate in solution (Warner 1957). Interestingly, the ImpU reaction products exhibit a strong signal at [oligomer – 18] that corresponds to the cyclic oligomer, while the ImpA and ImpG reaction products do not. The lack of observable cyclic oligomers for ImpA and ImpG, which should be major side-products from these reactions, could indicate that these cyclic oligomers are being strongly incorporated into stable aggregates. This is consistent with attribution of higher m/z peaks to aggregates of shorter cyclic and linear oligomers rather than single, longer linear oligomers.
MALDI-TOF MS spectra on steel AnchorChip using THAP matrix with ammonium citrate. a Montmorillonite-catalyzed ImpA polymerization products [m/z values: 3-mer (1004.7), 4-mer (1333.9), 5-mer (1663.1), 6-mer (1992.3), 7-mer (2321.5), 8-mer (2650.8)]. b Montmorillonite-catalyzed ImpG polymerization products [m/z values: 3-mer (1052.7), 4-mer (1397.9), 5-mer (1743.1), 6-mer (2088.3), 7-mer (2433.5)]. c Montmorillonite-catalyzed ImpU polymerization products [m/z values: 3-mer (935.7), 4-mer (1241.9), 5-mer (1548.1), 6-mer (1854.3), 7-mer (2160.5), 8-mer (2466.8), 9-mer (2773.0), 10-mer (3079.2)]. For all linear oligomers the following peaks are also observable: cyclic oligonucleotide [oligomer–18], sodium adducts [oligomer + 23.0 (Na+)–1.0 (H+)], potassium adducts [oligomer + 39.1 (K+)–1.0 (H+)]
Additionally, previous studies of montmorillonite-catalyzed RNA polymerization reactions utilizing ImpA have shown significantly longer polymerization products when analyzed by MALDI-TOF MS compared to HPLC (Zagorevskii et al. 2006). As noted above, this discrepancy was attributed to the lower limits of detection afforded by MALDI-TOF MS. Interestingly, the mass spectra in that study are very similar to those in the present work, but the previous peak assignments are shifted by (−18n) m/z units relative to ours. This shift in m/z assignment is possibly due to differences in calibration methods or to the previous study’s using the lower resolution linear mode MALDI-TOF MS spectra for oligomer characterization. Alternatively, their experimental conditions could be generating peaks not seen in this study.
While further work is needed to determine the source of the [oligomer + (18)n] peaks in our results, it is clear that the use of high resolution spectra and careful calibration are essential for accurate interpretation of MALDI-TOF mass spectral results for abiotic RNA polymerization reactions. We are currently pursuing studies to definitively assign the [oligomer + (18)n] peaks. Meanwhile, awareness of the uncertain origin of such peaks is essential in order to avoid their possible misattribution to single, linear polyA polymerization products.
Conclusions
Demonstration of abiotic polymerization of ribonucleotides under plausible prebiotic Earth conditions has become an increasingly active area in RNA world research. Recent reports that montmorillonite-catalyzed reactions may generate much larger products than previously reported (Zagorevskii et al. 2006), and that abiotic polymerization of cyclic monophosphate nucleotides may occur in the absence of a catalyst (Costanzo et al. 2011, 2012), are potentially important for the origin of an RNA world. However, the success of these approaches requires accurate analysis of the reaction products. As demonstrated in the present work using MALDI-TOF MS, non-covalent aggregation of smaller cyclic and linear oligonucleotides with each other, with monomeric ribonucleotides, or with small oligonucleotide contaminants in the reactant preparations offers a plausible alternative explanation for the mass spectral results obtained for abiotic polymerization products. Gel electrophoresis is of limited value as an independent method of verification since it is subject to many of the same ambiguities as MALDI-TOF MS in distinguishing between aggregation and polymerization. Precautions such as zip tipping and adjusting the MALDI matrix to basic pH can minimize the formation of adducts and aggregates, yet care must still be taken in calibration and interpretation of the mass spectra to consider all possible sources of the peaks, including both abiotic polymerization and other processes such as aggregation and adduct formation.
References
Banin A, Lawless JG, Mazzurco J, Church FM, Margulies L, Orenberg JB (1985) pH profile of the adsorption of nucleotides onto montmorillonite II. Adsorption and desorption of 5′-AMP in iron-calcium montmorillonite systems. Orig Life Evol Biosph 15:89–101
Brahms J, Michelson AM, Van Holde KE (1966) Adenylate oligomers in single- and double-strand conformation. J Mol Biol 15:467–488
Cassidy LM, Dong Y, Joshi PC, Aldersley MF, Ferris JP, McGown LB (2011) Signal enhancement of abiotically-synthesized RNA oligonucleotides and other biopolymers using unmodified fused silica in MALDI-MS. J Am Soc Mass Spectrom 22:1100–1104
Castleberry CM, Chou C, Limbach PA (2008) UNIT 10.1 matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of oligonucleotides. Curr Protoc Nucleic Acid Chem 10.1.1-10.1.21
Costanzo G, Pino S, Ciciriello F, Di Mauro E (2009) Generation of long RNA chains in water. J Biol Chem 284:33206–33216
Costanzo G, Pino S, Botta G, Saladino R, Di Mauro E (2011) May cyclic nucleotides be a source for abiotic RNA synthesis? Orig Life Evol Biosph 41:559–562
Costanzo G, Saladino R, Botta G, Giorgi A, Scipioni A, Pino S, Di Mauro E (2012) Generation of RNA molecules by a base-catalysed click-like reaction. ChemBioChem 13:999–1008
Davis J (2004) G-quartets 40 years later: from 5′-GMP to molecular biology and supramolecular chemistry. Angew Chem Int Ed 43:668–698
Distler AM, Allison J (2001) 5-methoxysalicylic acid and spermine: a new matrix for the matrix-assisted laser desorption/ionization mass spectrometry analysis of oligonucleotides. J Am Soc Mass Spectrom 12:456–462
Engelhart AE, Hud NV (2010) Primitive genetic polyers. Cold Spring Harb Perspect Biol 2:a002196
Ferris JP (2005) Mineral catalysis and prebiotic synthesis: montmorillonite-catalyzed formation of RNA. Elem 1:145–149
Ferris JP (2006) Montmorillonite-catalysed formation of RNA oligomers: the possible role of catalysis in the origins of life. Philos Trans R Soc London B Biol Sci 361:1777–1786
Ferris JP, Ertem G (1993) Montmorillonite catalysis of RNA oligomer formation in aqueous solution. A model for the prebiotic formation of RNA. J Am Chem Soc 115:12270–12275
Gesteland RF, Cech TR, Atkins JF (eds) (2006) The RNA World, 3rd Edn, Cold Spring Harbor Laboratory Press, New York
Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S (1983) The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35:849–857
Hansma HG (2010) Possible origin of life between mica sheets. J Theor Biol 266:175–188
Hazen RM, Sverjensky DA (2010) Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harb Perspect Biol 2:a002162
Horowitz ED, Engelhart AE, Chen MC, Quarles KA, Smith MW, Lynn DG, Hud NV (2010) Intercalation as a means to suppress cyclization and promote polymerization of base-pairing oligonucleotides in a prebiotic world. Proc Natl Acad Sci 107:5288–5293
Joshi PC, Aldersley MF, Zagorevskii DV, Ferris JP (2012) A nucleotide synthesis without protecting groups using montmorillonite as catalyst. Nucleosides Nucleotides Nucleic Acids 31:536–566
Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR (1982) Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 31:147–157
Orgel L (1986) RNA catalysis and the origins of life. J Theor Biol 123:127–149
Pfeffer S, Lagos-Quintana M, Tuschl T (2005) Cloning of Small RNA Molecules. Curr Protoc Mol Biol 26.4.4
Pieles U, Zürcher W, Schār M, Moser H (1993) Matrix-assisted laser desorption ionization time-of-flight mass spectrometry: a powerful tool for the mass and sequence analysis of natural and modified oligonucleotides. Nucleic Acids Res 21:3191–3196
Pino S, Ciciriello F, Costanzo G, Di Mauro E (2008) Nonenzymatic RNA ligation in water. J Biol Chem 283:36494–36503
Rich A, Davies DR, Crick FHC, Watson JD (1961) The molecular structure of polyadenylic acid. J Mol Biol 3:71–86
Sarracino D, Richert C (1996) Quantitative MALDI-TOF MS of oligonucleotides and a nuclease assay. Bioorg Med Chem Lett 21:2543–2548
Sutherland JD (2010) Ribonucleotides. Cold Spring Harb Perspect Biol 2:a005439
Wang S, Kool ET (1994) Circular RNA oligonucleotides. Synthesis, nucleic acid binding properties, and a comparison with circular DNAs. Nucleic Acids Res 22:2326–2333
Warner RC (1957) Studies on polynucleotides synthesized by polynucleotide phosphorylase. J Biol Chem 229:711–724
Zagorevskii DV, Aldersley MF, Ferris JP (2006) MALDI analysis of oligonucleotides directly from montmorillonite. J Am Soc Mass Spectrom 17:1265–1270
Zhu YF, Taranenko NI, Allman SL, Martin SA, Haff L, Chen CH (1996) The effect of ammonium salt and matrix in the detection of DNA by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 10:1591–1596
Acknowledgments
This research was funded by the National Aeronautics and Space Administration Astrobiology Institute through the New York Center for Astrobiology at Rensselaer Polytechnic Institute (Grant NNA09DA80A).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 65.3 kb)
Rights and permissions
About this article
Cite this article
Burcar, B.T., Cassidy, L.M., Moriarty, E.M. et al. Potential Pitfalls in MALDI-TOF MS Analysis of Abiotically Synthesized RNA Oligonucleotides. Orig Life Evol Biosph 43, 247–261 (2013). https://doi.org/10.1007/s11084-013-9334-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-013-9334-5