Abstract
A model describing oxidation kinetics considering simultaneous oxidation and volatilization of external oxide scales was integrated with a CALPHAD-based coupled thermodynamic and kinetic approach to predict the oxidation-induced lifetime of foils of alloys 625 and 120. Long-term exposures in flowing air +10% \({\hbox {H}_2\hbox {O}}\) at 700 and 800 \(^\circ\)C under two flow velocities were used to validate the modeling results. The time to a critical Cr concentration of 10 wt% at the oxide/alloy interface, \(t_{\text {10}}\), was shown to correlate with the breakdown of the \({\hbox {Cr}_2\hbox {O}_3}\) scale for the studied experimental conditions. Finally, the modeling approach was extended to consider time-dependent oxidation and volatilization rate constants to develop a more appropriate lifetime criterion for microturbine recuperator foils.
Similar content being viewed by others
Introduction
One strategy to increase the electrical efficiency of recuperated microturbines is to operate at higher temperatures, such that the recuperator inlet temperature increases to \(\ge\) 700 \(^\circ\)C (currently 600–650 \(^\circ\)C) [1]. However, such an increase in operating temperatures was shown to accelerate the detrimental effect of water vapor on the degradation of previously used stainless steel recuperator materials (e.g. type 347 steel) [2,3,4]. The presence of water vapor in the microturbine’s exhaust gas atmosphere [3] induces volatilization of the \({\hbox {Cr}_2\hbox {O}_3}\) scale into \({\hbox {CrO}_2\hbox {(OH)}_2}\) [5, 6] which hastens the breakaway oxidation of austenitic steels, in high oxygen partial pressures [7, 8]. Therefore, more expensive, oxidation and creep resistant \({\hbox {Cr}_2\hbox {O}_3}\)-forming Ni-Fe-based alloys (such as alloy HR120) and Ni-based alloys (such as alloy 625) are now being used [9, 10].
The \({\hbox {Cr}_2\hbox {O}_3}\) scale formed on the surface of these alloys is also prone to volatilization but the extent of degradation of the specimens depends on experimental parameters (temperature, gas flow rate, alloy composition) [11,12,13,14,15,16]. The formation of Fe-rich oxide nodules on the surface of alloy 709 foils started earlier at 800 \(^\circ\)C (7000 h) than at 650 \(^\circ\)C (10,000 h) after exposure in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) (velocities of 1.7 cm s\(^{-1}\) and 2.0 cm s\(^{-1}\) at 650 and 800 \(^\circ\)C, respectively) [11, 12]. Additionally, greater Cr loss was observed (30% of initial Cr content) in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) than in dry air (10% of initial Cr content) for alloy 625 foil at 800 \(^\circ\)C although no breakdown of the \({\hbox {Cr}_2\hbox {O}_3}\) scale was observed [11]. A higher gas flow velocity (v = 0.76 cm s\(^{-1}\)) led to greater measured mass loss for Ni-based alloy 230 after 400 h of exposure at 760 \(^\circ\)C in air + 37–38% \({\hbox {H}_2\hbox {O}}\) than in lower gas flow velocity (v = 0.19 cm s\(^{-1}\)) while no breakdown of the \({\hbox {Cr}_2\hbox {O}_3}\) scale was observed after 2000 h of exposure under the low velocity gas [13]. At 900 \(^\circ\)C, the breakdown of the \({\hbox {Cr}_2\hbox {O}_3}\) scale formed on the surface of alloy 625 foils was observed to occur after only 1000 h in high gas velocity air + 6% \({\hbox {H}_2\hbox {O}}\) (70 cm s\(^{-1}\)) but was not observed at 5 cm s\(^{-1}\) [14]. Finally, the breakdown of the \({\hbox {Cr}_2\hbox {O}_3}\) scale into Fe and Ni-rich oxides was observed on the surface of 1 mm thick alloy 690 (Ni-30Cr-10Fe) after 200 h exposure in flowing air + 20% \({\hbox {H}_2\hbox {O}}\) (31.6 cm s\(^{-1}\)) at 800 \(^\circ\)C [15].
Because of the competing mechanisms of oxidation and evaporation, long-term experiments are often necessary to evaluate and compare the oxidation lifetime of different alloys exposed in various experimental conditions. However, such approaches are costly and time-consuming. Semi-quantitative models predicting Cr loss of \({\hbox {Cr}_2\hbox {O}_3}\)-forming Ni-based alloys in various wet air environments and conditions have been reported in the literature [12,13,14, 17,18,19]. Duan and co-workers adapted the cyclic oxidation model (COSP) [20] to predict the mass change and the time to critical Cr content for the oxidation and spallation behavior of alloy 230 specimens (0.2–0.5 mm thick), oxidized for 3000 h at 950, 1000 and 1050 \(^\circ\)C in dry air (10 and 48 h cycles) [21]. Two parameters were adjusted, \({\hbox {Q}_0}\) (spalling constant) and \(\theta\) (considering additional oxygen ingress from the formation of microtensile cracks in the oxide layer during repetitive specimen heating). Good agreement was found between experimental and predicted times to a failure criteria of 10 wt% Cr at the alloy oxide interface (\(t_{\text {10}}\)) for 0.2 mm thick H230 foils exposed 3000 h at 1000 and 1050 \(^\circ\)C, \({{Q}_0}\) = 0 cm\(^{2}\) mg\(^{-1}\) (only oxidation observed and considered in the model) and \(\theta\) = 0.0033 [21]. However, these modeling approaches [12,13,14, 17,18,19, 21] did not consider the combined oxidation and volatilization of the \({\hbox {Cr}_2\hbox {O}_3}\) scale. This shortcoming was addressed in a study of alloy 120 and 625 foils between 650 and 800 \(^\circ\)C in dry air and flowing air + 10% \({\hbox {H}_2\hbox {O}}\) (850 cm\(^{ 3}\) min\(^{-1}\)) where good agreement was found between experimentally and predicted total Cr losses before the breakdown of the protective \({\hbox {Cr}_2\hbox {O}_3}\) scale [22]. One issue was that after the formation of mixed Fe, Cr and Ni-rich oxides, the oxidation and volatilization model strongly underestimated the Cr loss from both materials [22]. Consideration of simultaneously occurring diffusion processes [23, 24] and consequent microstructural changes [25, 26] was beyond the scope of this previous combined model. Furthermore, the impact of wall thickness loss on the Cr depletion, especially critical for thin components, was not addressed. Coupled thermodynamic and kinetic models employing assessed CALPHAD-based thermodynamic and kinetic data have been previously shown to be useful to describe oxidation-induced microstructural changes and predict corresponding material lifetimes [26,27,28].
The aim of this study was to combine a model describing the Cr loss kinetics, considering both oxidation and volatilization with the coupled thermodynamic-kinetic model to predict oxidation-induced lifetime of foil materials in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) as a function of time, temperature, foil thickness and flow velocities. The developed model is envisioned to be a valuable tool to help future material evaluation and selection for recuperated microturbines and other applications that involve these reactions and microstructural changes. The oxidation lifetime model was evaluated for two gas flow rates (850 and 5000 cm\(^{3}\) min\(^{-1}\)) and specimen thicknesses between 50 and 150 \(\upmu\)m at 700 and 800 \(^\circ\)C. Experimental results and characterizations of the combined oxidation and volatilization behavior of two alloy foils (625 and 120) exposed in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) at 700 and 800 \(^\circ\)C were used to validate the model [22].
Experimental Procedures
Rectangular foil specimens of Haynes\(^\circledR\) alloy 120 (82 ± 2 \(\upmu\)m thickness) and 625 (100 ± 7 \(\upmu\)m thickness) (analyzed compositions in Table 1) were placed in alumina boats and exposed in a resistively heated alumina tube furnace (diameter of 5.9 cm) for up to 10,000 h in dry air (100 and 500 h-cycles) at 700 and 800 \(^\circ\)C (alumina crucibles), as well as up to 30,000 h at 700 \(^\circ\)C and up to 10,000 h at 800 \(^\circ\)C in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) (850 cm\(^{3}\) min\(^{-1}\), 100 h-cycles) without crucibles [22]. Additional exposures for up to 10,000 h were conducted in the furnace at 700 \(^\circ\)C in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) (5000 cm\(^{3}\) min\(^{-1}\), 500 h-cycles) without crucibles. The resulting gas velocities for the three temperatures and two flow rates are reported in Table 2.
After exposure, specimens were Cu plated and mounted in conductive epoxy for cross-sectional characterizations including Scanning Electron (SE), Back-scattered Electron Microscopy (BSE) (SEM TESCAN model MIRA3), energy-dispersive X-ray spectroscopy (EDAX Octane Elect Super Silicon Drift Detector) and electron probe microanalysis (EPMA JEOL model 8200/8500).
Modeling Approach
A coupled thermodynamic and kinetic model [27,28,29] was used to predict compositional and microstructural changes in alloys 625 and 120. This model was implemented in a FORTRAN program allowing calculations to be run on parallel computing cores [28]. The required thermodynamic and kinetic data were extracted from the thermodynamic database TCNI8 and mobility database MOBNI4, respectively. Initial compositions of the alloys correspond to the values in Table 1. The phases relevant for each alloy such as 120 (\(\gamma\)-FCC, \({\hbox {M}_{23}}\) \({\hbox {C}_6}\)) and 625 (\(\gamma\)-FCC, \(\updelta\)- \({\hbox {Ni}_3}\)Nb, \({\hbox {M}_6}\)C and \({\hbox {M}_{23}}\) \({\hbox {C}_6}\)) were considered while diffusion was assumed to occur solely in the \(\gamma\)-FCC matrix. A planar geometry was discretized with a geometric mesh (finer at the alloy surface) that was adapted at each time step to account for the loss of wall thickness during oxidation and volatilization. Assuming exclusive formation of \({\hbox {Cr}_2\hbox {O}_3}\), the Cr-flux (left boundary condition for profiles shown below) values were determined by solving the para-linear oxidation behavior [17] described in [30] with a MATLAB partial differential equation solver [31]. A detailed description of the procedure can be found elsewhere [22]. The assumption of symmetry at the center of the samples provided the second spatial boundary condition of zero flux for all elements (right boundary condition).
Results
Mass Change
Figure 1 shows the mass change of 625 (Fig. 1a) and 120 (Fig. 1b) specimens after 10,000 h of exposure in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) at 700 \(^\circ\)C and for two gas flow rates of 850 and 5000 cm\(^{3}\) min\(^{-1}\) (referred to as low flow rate (LFR) and high flow rate (HFR), respectively). The LFR results were previously reported [22]. When available, the mass change data were averaged and the standard errors of the mean are shown in Fig. 1.
As expected, larger mass losses were observed in HFR than in LFR for alloy 625 at 700 \(^\circ\)C (Fig. 1a). In addition, a slight deviation from linear mass losses was observed after about 4,200 h of exposure in HFR but was not observed in LFR (Fig. 1a). The higher gas flow rate did not result in larger mass losses for the 120 specimens during the first 4000 h at 700 \(^\circ\)C (Fig. 1b). In addition, larger mass change variations were observed in HFR than in LFR. As a result, two averaged mass change curves from two sets of three foils are shown in Fig. 1b in HFR where rapid mass gains of 0.1\(-\)0.4 mg cm\(^{2}\) between 4000 and 6000 h were observed. A sudden mass increase was also observed in LFR, but after a longer time (around 10,000 h) than in HFR. After the mass gain, the specimen mass varied minimally (between 0.05 and 0.1 mg cm\(^{2}\)) for exposures up to 30,000 h.
Oxide Microstructure
For both the LFR and HFR 625 specimens, the EDS maps in Fig. 2a revealed the formation of a Cr-rich scale with Nb- and Cr-rich oxide incorporations after 10,000 h exposures at 700 \(^\circ\)C. In addition, Nb-enrichment was observed at the alloy-oxide interface and Fe-rich oxides (Fig. 2a) and Fe, Ni-rich oxides (Fig. 2b) were observed on top of the Cr-rich scale at some locations.
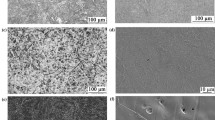
For the alloy 120 specimens, a Cr- and Mn-rich scale was observed after 5000 h at 700 \(^\circ\)C in LFR (Fig. 3a, note higher magnification of images in Fig. 3a compared to Figs. 2and 3b). However, after 5000 h in HFR, a breakdown of the Cr- and Mn-rich scale resulted in the formation of duplex-structured Fe, Cr and Ni-mixed oxide nodules (Fig. 3b). A time series of images in Fig. 4 shows that a thin Cr- and Mn-rich oxide was maintained in HFR for 3500 h but nodules were observed after 5000 h and continued to grow and covered the entire surface after 10,000 h at 700 \(^\circ\)C (Fig. 4c). In LFR, the transition from Mn and Cr-rich oxides to Fe, Cr and Ni-mixed oxides was observed to occur after 5000 h and characterization of these specimens has been reported elsewhere [22].
Compositional Changes in the Underlying Alloys
Oxidation-induced Cr depletion profiles were measured by EDS and EPMA in the matrix below the external Cr-rich oxide scales on both alloys. An average of three measured profiles and the calculated Cr depletion profile are reported in Fig. 5a after 10,000 h in LFR and HFR at 700 \(^\circ\)C for alloy 625 specimens. The measurements of the Cr concentration at the alloy-oxide interface were affected by the \(\delta\)-\({\hbox {Ni}_3\hbox {Nb}}\) enrichment below the \({\hbox {Cr}_2\hbox {O}_3}\) oxide (Fig. 2b) which resulted in greater relative error for the EDS measurements, mostly in HFR. The \(\delta\)-\({\hbox {Ni}_3\hbox {Nb}}\) did not form a continuous layer below the oxide scale (Fig. 2a) in LFR which resulted in a smaller relative error in the average Cr concentration measurements at the alloy-oxide interface. The Cr depletion depth was measured from the alloy-oxide interface until the measured Cr concentration reached 10% relative of the initial Cr concentration in the alloy.
Experimentally, similar Cr depletion depths were measured in HFR and LFR (7 ± 2 \(\upmu\)m) for alloy 625 at 700 \(^\circ\)C after 10,000 h (Fig. 5a). However, the concentration at the alloy-oxide interface was greater in LFR, 13 ± 3 wt%, than in HFR, 3 ± 3 wt%. Predicted Cr depletion depths were in agreement with experimental measurements in both environments. The model successfully predicted a lower Cr concentration at the alloy-oxide interface in HFR than in LFR (Fig. 5a).
For alloy 120, the experimental and calculated Cr concentration profiles are shown after 5000 h (Fig. 5b) and 10,000 h of exposure (Fig. 5c) in LFR and HFR at 700 \(^\circ\)C. Similar Cr depletion depths were measured in HFR and LFR after 5000 h (Fig. 5b). However, the measured Cr concentration at the alloy oxide interface was lower in HFR (10 ± 1 wt%) than in LFR (15 ± 1 wt%). After 10000 h, the measured Cr depletion depths increased and the Cr concentration at the alloy oxide interface decreased to 10 ± 1 wt% and 9 ± 3 wt% in LFR and HFR, respectively (Fig. 5c). In addition, the measured Cr depletion depth was larger in HFR (10 ± 3 \(\upmu\)m) than in LFR (5 ± 1 \(\upmu\)m) after 10,000 h at 700 \(^\circ\)C (Fig. 5c). Calculated Cr alloy-oxide interfacial concentrations were in agreement with experimental measurements after 5000 h and 10,000 h at 700 \(^\circ\)C in both environments. The model correctly predicted the Cr depletion depth after 5000 h in both environments but underestimated the Cr depletion depth after 10,000 h in HFR (about 5 \(\upmu\)m depletion was predicted while about 10 ± 3 \(\upmu\)m was measured in Fig. 5c).
Discussion
Oxidation Behavior
The oxidation behavior of alloy 625 has been studied in various atmospheres [14, 23, 32, 33]. The early stage oxidation behavior of alloy 625 was studied in oxygen partial pressures of 0.12 kPa and 101 kPa between 600 and 1250 \(^\circ\)C for durations less than 150 min [32]. Between 600 and 927 \(^\circ\)C, XPS and AES characterizations indicated that the oxide scale consisted almost exclusively of \({\hbox {Cr}_2\hbox {O}_3}\). Above 1150 \(^\circ\)C, Nb- and Ti-rich oxides were also formed in addition to \({\hbox {Cr}_2\hbox {O}_3}\) [32]. The enrichment of \(\updelta-\hbox {Ni}_3\hbox {Nb}\) was observed at the oxide-alloy interface in various atmospheres [14, 23, 34]. Huczkowski et al. [14] confirmed with EDS and EBSD analyses that the \(\updelta-\hbox {Ni}_3\hbox {Nb}\) enrichment at the alloy-oxide interface led to its subsequent oxidation into \({\hbox {NbCrO}_4}\) at the leading edge of 625 foil after 1000 h of exposure in flowing air + 6% \({\hbox {H}_2\hbox {O}}\) (0.7 m s\(^{-1}\)) at 900 \(^\circ\)C. The observed larger mass losses within the present study (Fig. 1) in HFR as compared to LFR correspond to the expected greater volatilization rate of the \({\hbox {Cr}_2\hbox {O}_3}\) in HFR than in LFR (due to the \({\hbox {CrO}_2(\hbox {OH})_{2(\hbox {g})}}\) formation), in agreement with what is predicted from mass transfer theory [12, 13]. These results and EDS elemental maps shown in Fig. 2 suggested the formation of a \({\hbox {Cr}_2\hbox {O}_3}\) scale on the surface of alloy 625 and partial oxidation of the Nb-enriched layer at the oxide/alloy interface into \({\hbox {NbCrO}_4}\) both in LFR and HFR, in agreement with the literature observations [14, 23, 32, 33].
For alloy 120, EDS elemental maps in Fig. 3 suggested the initial formation of \({\hbox {Cr}_2\hbox {O}_3}\) oxide underneath a \({\hbox {MnCr}_2\hbox {O}_4}\) spinel oxide as reported in the literature for both this alloy [22, 35] and for many similar alloys [4, 11, 36,37,38]. The more rapid transition from \({\hbox {Cr}_2\hbox {O}_3}\) scale formation (protective oxidation behavior) to Fe, Cr and Ni mixed oxides (less-protective oxidation behavior) in HFR than in LFR is also in agreement with predictions from mass transfer theory [12, 13]. A similar transition from protective to non-protective oxidation behavior occurred more rapidly in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) compared to dry air exposure at 800 \(^\circ\)C [22]. In contrast to alloy 625, larger mass losses were not observed in HFR than in LFR (Fig. 1). The increased oxidation kinetics of the Fe, Cr and Ni mixed oxides that started to form before 3,500 h likely compensated for the increased mass loss in the high velocity gas flow conditions. Alternatively, the mixed oxides with underlying \({\hbox {Cr}_2\hbox {O}_3}\) scale formed on the alloy 120 specimens could result in lower oxidation and volatilization rates [22] compared to the \({\hbox {Cr}_2\hbox {O}_3}\) scale formed on alloy 625 specimens at 700 \(^\circ\)C. After the formation of the oxide nodules, the period of very low mass gain could include a small amount of spallation.
Differences Between Experimental and Predicted Compositional Changes in the Underlying Alloys
For alloy 120, the discrepancy between experimental and predicted Cr depletion depths after 10,000 h in HFR at 700 \(^\circ\)C (Fig. 5c) can be explained by the presence of the Fe, Cr and Ni-mixed oxides on both sides of the foil (4c). The transition from a Cr- and, Mn-rich oxide scale to a faster growing Fe, Cr and Ni-mixed oxides will induce larger Cr consumption and, therefore, increased depletion in the underlying alloy. This change in oxidation behavior was not considered in the model (only \({\hbox {Cr}_2\hbox {O}_3}\) oxidation and volatilization rates were considered as reported in “Modeling approach”section) and therefore underestimated the Cr depletion depths after the transition. Larger Cr losses were measured after the breakdown of the \({\hbox {Cr}_2\hbox {O}_3}\) scale for alloy 120 foils exposed in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) at 700 and 800 \(^\circ\)C [22].
Experimental and predicted Cr concentration profiles in the underlying alloy were used to determine the critical Cr concentration at the alloy-oxide interface at which the breakdown of the protective \({\hbox {Cr}_2\hbox {O}_3}\) scale is observed. In the literature, this value was evaluated to be about 16 wt% Cr for 20Cr-25Ni austenitic steel coupons oxidized in 1–2 vol% CO with about 200 vpm \({\hbox {CH}_4}\) and \({\hbox {H}_2\hbox {O}}\) between 750 and 900 \(^\circ\)C [39, 40]. For Ni-based alloys, the critical Cr concentration needed to maintain \({\hbox {Cr}_2\hbox {O}_3}\) formation was estimated to be around 10 wt% at 1000 \(^\circ\)C in dry oxygen atmosphere at a pressure of 200 Torr [41]. Similarly, a critical Cr concentration of about 9 wt% was observed to lead to breakaway oxidation at the edges of 0.2 mm thick Ni-based 230 foil after 2000 h oxidation in air at 1050 \(^\circ\)C [21]. The fact that Fe, Cr and Ni mixed oxide nodules were observed at some locations on the surface of alloy 120 specimens when about 10 wt% Cr was reached at the alloy-oxide interface after 5000 h at 700 \(^\circ\)C (Fig. 5b) suggests that 10 wt% Cr is a reasonable estimation of the critical Cr concentration required to maintain \({\hbox {Cr}_2\hbox {O}_3}\) formation in case of the alloy 120 specimens at 700 \(^\circ\)C in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) in HFR and LFR. As mentioned previously, the Cr concentration measurements and calculations for alloy 625 specimens were strongly affected by the \(\delta\)-\({\hbox {Ni}_3\hbox {Nb}}\) enrichment at the alloy-oxide interface. This resulted in measured and calculated Cr concentrations at the interface substantially below 10 wt% (3 ± 3 wt% in Fig. 5a) while the oxide scale was still mainly \({\hbox {Cr}_2\hbox {O}_3}\) (Fig. 2b) after 10,000 h in HFR at 700 \(^\circ\)C. However, the local thicker Fe, Cr and Ni-mixed oxides were observed after 10,000 h at HFR than at LFR (Fig. 2) in agreement with the higher calculated and measured Cr interfacial concentrations in LFR (13 ± 3 wt%) than in HFR (3 ± 3 wt%) in Fig. 5a. As a result, a critical Cr concentration of 10 wt% also seems reasonable for alloy 625 at 700 \(^\circ\)C flowing air + 10% \({\hbox {H}_2\hbox {O}}\).
Establishment of Oxidation Lifetime Diagrams
The good agreement observed between experimental and calculated Cr profiles presented in “Compositional changes in the underlying alloys”section allowed the estimation of the time required to reach the critical Cr concentration of 10 wt% at the oxide-alloy interface in order to maintain \({\hbox {Cr}_2\hbox {O}_3}\) for both alloys 625 and 120 in wet air environments. Oxidation lifetime diagrams, previously proposed by Duan et al. [21], were constructed by plotting the calculated total metal loss (both sides of the foil specimens) as function of time for both alloys at 700 and 800 \(^\circ\)C in HFR, LFR and dry air atmospheres (Figs. 6 and 7). The calculated time to 10 wt% Cr, \(t_{\text {10}}\), was also reported on the same diagram in order to provide a chemical lifetime criteria corresponding to the expected breakdown of the \({\hbox {Cr}_2\hbox {O}_3}\) scale as established earlier and in agreement with prior work [21, 41].
Oxidation lifetime diagrams: calculated metal losses as function of time and calculated \(t_{\text {10}}\) (circles) after exposure at 700 \(^\circ\)C in dry air, flowing air + 10% \({\hbox {H}_2\hbox {O}}\) at 850 cm\(^{3}\) min\(^{-1}\) and flowing air + 10% \({\hbox {H}_2\hbox {O}}\) at 5000 cm\(^{3}\) min\(^{-1}\) of alloy studied 625 (100 \(\upmu\)m) foils and HR120 (85 \(\upmu\)m) foils
In Fig. 6, a \({\hbox {Cr}_2\hbox {O}_3}\) scale was predicted to continue to form beyond 10,000 h of exposure for both alloys 625 and 120 (Fig. 6) in dry air, in agreement with experimental observations of alloy 625 and 120 foils exposed in dry air at 700 \(^\circ\)C [22]. In addition, \(t_{\text {10}}\) was calculated to be reached earlier for alloy 120 than alloy 625 in LFR and HFR at 700 \(^\circ\)C.
For alloy 625 in wet air, the Cr concentration at the alloy-oxide interface was measured to be 13 ± 3 wt% after 10,000 h of exposure at 700 \(^\circ\)C in LFR (Fig. 5a) which suggested that \(t_{\text {10}}\) was not yet reached experimentally although the model predicted \(t_{\text {10}}\) = 9400 h (Fig. 6). Some Nb-rich oxides were incorporated in the \({\hbox {Cr}_2\hbox {O}_3}\) scale and Fe-rich oxides were observed at some locations (Fig. 2a) therefore indicating that \(t_{\text {10}}\) could have been reached at these locations. The model predicted the time to \(t_{\text {10}}\) of 8,110 h at 700 \(^\circ\)C in HFR suggesting a transition to the formation of oxides other than \({\hbox {Cr}_2\hbox {O}_3}\) before 10,000 h. Although the oxide on the surface of alloy 625 was mainly \({\hbox {Cr}_2\hbox {O}_3}\) after 10,000 h in HFR at 700 \(^\circ\)C, the local formation of Fe and Ni-rich oxides at a few locations (Fig. 2b) validates the model predictions (Fig. 6).
For alloy 120, \(t_{\text {10}}\) was also predicted to be reached earlier in HFR (7020 h) than in LFR (7814 h) (Fig. 6). Experimentally, only time ranges can be given regarding the experimental values of \(t_{\text {10}}\). In Fig. 1b, the transition from mass loss behavior to slight mass gain occurred earlier in HFR (between 3500 and 5000 h) than in LFR (between 5000 h and 10,000 h) in agreement with the model predictions. From BSE and EDS elemental maps the first Fe-rich oxide nodules, that were located above the grain boundaries, were also identified earlier in HFR (3500 h) than in LFR (10,000 h). This resulted in experimental variations in the interfacial Cr concentration until the nodules covered the entire surface of the H120 foil specimens up to 10,000 h both in HFR and LFR, as observed in Fig. 4. This variation cannot be considered by the 1D model, which does not consider accelerated grain boundary diffusion and therefore explains why the \(t_{\text {10}}\) value (7,020 h) was predicted after the apparition of the first Fe-rich nodules (3,500 h) and before the nodules covered the entire surface (10,000 h) in HFR (Fig. 4).
a Oxidation lifetime diagrams: calculated metal losses as function of time \(t_{\text {10}}\) after exposure at 800 \(^\circ\)C in dry air, flowing air + 10% \({\hbox {H}_2\hbox {O}}\) at 850 cm\(^{3}\) min\(^{-1}\) and flowing air + 10% \({\hbox {H}_2\hbox {O}}\) at 5000 cm\(^{3}\) min\(^{-1}\) of alloy studied 625 (100 \(\upmu\)m) foils and HR120 (85 \(\upmu\)m) foils. b Corresponding BSE images after 5000 and 10,000 h in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) at 850 cm\(^{3}\) min\(^{-1}\) and dry air
At 800 \(^\circ\)C, similar to the observations at 700 \(^\circ\)C, \(t_{\text {10}}\) was reached earlier for alloy 120 than alloy 625 in dry and wet air atmospheres (Fig. 7) in agreement with prior reports [22]. For alloy 625, the \({\hbox {Cr}_2\hbox {O}_3}\) scale was predicted to be maintained over 10,000 h in dry air in agreement with experimental results [22] and the BSE image in Fig. 7 after 10,000 h in dry air. A \(t_{\text {10}}\) value of 3,506 h was predicted in LFR at 800 \(^\circ\)C (Fig. 7). However, a \({\hbox {Cr}_2\hbox {O}_3}\) scale was still observed on the surface of the specimen after 5000 h in LFR at 800 \(^\circ\)C (BSE image in Fig. 7). Below the oxide scale, \(\updelta\)- \({\hbox {Ni}_3}\)Nb enrichment was also observed but did not form a continuous layer (BSE image in Fig. 7). This resulted in experimental variations of the Cr concentration at the alloy-oxide interface (8 and 11 wt% in Fig. 8a). This variation cannot be captured by the model which effectively considered a homogeneous, single crystal material and therefore predicted \(\updelta\)-\({\hbox {Ni}_3}\)Nb enrichment everywhere below the \({\hbox {Cr}_2\hbox {O}_3}\) scale (Fig. 8b). The fact that the \({\hbox {Cr}_2\hbox {O}_3}\) scale was observed all over the specimen surface after 5000 h at 800\(^\circ\)C, independently of the Cr concentration being below or above 10 wt%, suggest that, in addition to Cr diffusing from the alloy center towards the surface, Cr diffused laterally from greater to lower Cr concentrations in order to maintain the formation of the \({\hbox {Cr}_{2\hbox {O}3}}\). Again, this trend of lateral diffusion cannot be captured with the 1D modeling approach used in this study and therefore explains why the model predicted a shorter \(t_{\text {10}}\) than that experimentally observed (between 5000 and 10,000 h at 800 \(^\circ\)C in LFR).
For alloy 120, \(t_{\text {10}}\) was calculated to be reached after 10,000 h in dry air at 800 \(^\circ\)C which was in accordance with the local formation of Fe, Cr and Ni mixed oxide nodules on the surface after 10,000 h exposure in dry air at 800 \(^\circ\)C [22] and in the BSE image in Fig. 7. In LFR, a \(t_{\text {10}}\) value of 1,692 h was predicted by the model while nodules were sparsely observed on the surface of the alloy 120 after 5000 h exposure at 800 \(^\circ\)C. At this condition, no specimen was tested between 1000 h (where only \({\hbox {Cr}_2\hbox {O}_3}\) formation was observed) and 5000 h.
Although, \(t_{\text {10}}\) was reached earlier for alloy 120 than for alloy 625 for all experimental and predicted conditions, the values did not differ greatly (less than 2000 h in wet air atmospheres, substantially even less in some case, Figs. 6 and 7). In accordance with experimental foil thickness measurements, the predictions were made considering foils of two different thicknesses (100 \(\upmu\)m and 85 \(\upmu\)m for alloys 625 and 120, respectively). To rigorously compare the oxidation lifetime of alloy foils with various elemental compositions, it is necessary to compare similar thicknesses. Therefore, additional predictions were made at 800 \(^\circ\)C in HFR for two foil thicknesses 50 and 150 \(\upmu\)m. The results are reported in Fig. 9. In agreement with the results presented in Figs. 6 and 7, lower \(t_{\text {10}}\) values were calculated for alloy 120 than for alloy 625 for both thicknesses. Furthermore, the difference was more noticeable for 150 \(\upmu\)m foils than 50 \(\upmu\)m foils, where the combined effect of low oxidation rate constant and lower Cr mobility in FCC (Table 3) doubles the predicted oxidation lifetime for 625 foil when its thickness is increased from 50 to 150 \(\upmu\)m compared to less than 10% gain in time to breakdown when a 120 foil is similarly thickened.
Oxidation Lifetime of Microturbine Recuperator Foils
It is important to mention that the calculated total metal losses based on \({\hbox {Cr}_2\hbox {O}_3}\) oxidation and volatilization remained equal or lower than 10 \(\upmu\)m before \(t_{\text {10}}\) was reached for both alloys in two gas flow rates and at the two studied temperatures (Figs. 6 and 7). This corresponded to 12 and 10% total metal loss for alloys 120 and 625, respectively. Therefore, in terms of recuperator design and oxidation and mechanical lifetime, it is unlikely that the \(t_{\text {10}}\) criteria correspond to an end of recuperator’s lifetime in service at 700 or 800 \(^\circ\)C in high and low flowing air + 10% \({\hbox {H}_2\hbox {O}}\). Therefore, in this study, the oxidation lifetime model was extended to implement the growth and volatilization of Fe, Cr and Ni-mixed oxides after the breakdown of the \({\hbox {Cr}_2\hbox {O}_3}\) scale.
Extended Model Description and Parameters
The model previously presented [22] was used and extended with time dependent oxidation rate constants and volatilization rate constants. The \(t_{\text {10}}\) values calculated in this study were used to determine the transition from \({\hbox {Cr}_2\hbox {O}_3}\) formation to mixed oxides. No literature data have been found on the exact oxidation rate constant of Fe-, Cr- and Ni-rich or Nb- and Cr-rich oxides between 700 and 800 \(^\circ\)C in wet air environments. Similarly, no literature data were found on the volatility of these oxides. Therefore, the oxidation rate constants were estimated from the thicknesses measured in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) (850 cm\(^{3}\) min\(^{-1}\)) at 700 and 800 \(^\circ\)C [22]. The partial pressure of mixed oxide volatile species was estimated averaging those between pure \({\hbox {CrO}_2\hbox {(OH)}_2}\) [5] and \({\hbox {Fe(OH)}_2}\) [42]. The oxidation rate constant at 750 \(^\circ\)C was calculated based on the observed ratio between the oxidation rate constant of \({\hbox {Cr}_2\hbox {O}_3}\) and Fe, Cr and Ni mixed oxides at 700 and 800 \(^\circ\)C, hence between 10 and 17 times greater than the oxidation rate constant of the \({\hbox {Cr}_2\hbox {O}_3}\) at these temperatures as reported in Table 4. All values are reported in Table 4 and partial pressures in Table 5.
The resulting calculated lifetime diagram is reported in Fig. 10 for alloy 120 and 625 foils between 700 and 800 \(^\circ\)C in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) at 850 cm\(^{3}\) min\(^{-1}\). Reported experimental total metal losses (both sides) were determined using the Cr depletion profiles reported previously assuming homogeneous external oxidation of \({\hbox {Cr}_2\hbox {O}_3}\) and \({(\hbox {Nb,Fe})\hbox {Cr}_2\hbox {O}_4}\) when observed [22]. The effects of microstructure (grain boundaries) are not considered in the model and therefore the metal loss due to internal oxidation at grain boundaries and pores formed below the oxide scale that could contribute to the overall metal loss of the studied foils is not accounted for. Calculated total metal losses are determined based on Cr, Nb and Fe loss from \({\hbox {Cr}_2\hbox {O}_3}\) and \({(\hbox {Nb,Fe})\hbox {Cr}_2\hbox {O}_4}\) formation. Finally, the recuperator end of time was defined when about one third of the foil thickness is lost due to the combined effect of oxidation and volatilization before a required lifetime of 30,000 h.
In Fig. 10a, the calculated metal loss was less than 5 \(\upmu\)m and agreement with experimental measurements at 700 \(^\circ\)C for alloy 120 specimens. At 750 \(^\circ\)C, using the estimated parameters from Tables 4 and 5, lower metal loss was predicted by the model than experimentally measured at 700 \(^\circ\)C for alloy 120 specimens. This suggested an underestimation of the volatilization and oxidation rates at 750 \(^\circ\)C. For this alloy, the total metal loss becomes significant at 800 \(^\circ\)C due to the rapid growth of mixed oxides compromising its use at this temperature which is in agreement with prior observations [22] that were also reported in Fig. 10a. For alloy 625, good agreement was found between predicted and experimental metal loss using Cr loss from EPMA profiles [22] after 10,000 h (Fig. 10b). However, predictions underestimated metal losses when the experimental metal loss was determined from the thickness of the EPMA line scans after 10,000 h (considering a initial foil thickness of 120 \(\upmu\)m) (Fig. 10b). In addition, affected metal zones deeper in the alloy, such as heterogeneous internal oxidation at grain boundaries and the formation of pores, were not considered in the model which did not consider internal oxidation and alloy grain boundaries. These additional degradation mechanisms could compromise the mechanical integrity of the recuperator foils and could be accounted for using phase field modeling [43]. Furthermore, the impact of the optimized geometry of the microturbine recuperator foils [44] on the oxidation lifetime cannot be studied with the current 1D model. The influence of the presence of multiple corners on the elemental depletions could only be evaluated with the extension of the model to a 2D geometry. Finally, the impact of higher hydrogen levels on the oxidation and volatilization lifetime of future hydrogen-fueled microturbines needs to be studied [45, 46]. Validation of the presented model using a higher \({\hbox {H}_2\hbox {O}}\) atmosphere (air + 60% \({\hbox {H}_2\hbox {O}}\)) is currently underway.
Experimental (symbols) [22] and estimated lifetime of microturbine’s recuperator (lines) foils or metal loss of the (a) 120 or (b) 625 foil as function of time in flowing air + 10% \({\hbox {H}_2\hbox {O}}\) (850 cm\(^{3}\) min\(^{-1}\)) between 700 and 800 \(^\circ\)C
Conclusions
An oxidation and volatilization model was implemented into a CALPHAD-based coupled thermodynamic and kinetic model to predict oxidation and volatilization-induced Cr loss and lifetime (critical Cr content to form \({\hbox {Cr}_2\hbox {O}_3}\), \(t_{\text {10}}\)) of two alloy foils 625 and 120 after exposure in air + 10% \({\hbox {H}_2\hbox {O}}\) (850 and 5000 cm\(^{3}\) min\(^{-1}\)) at 700 and 800 \(^\circ\)C. In agreement with experimental characterization, the model predicted greater Cr losses and reduced lifetime (\(t_{\text {10}}\)) in high flow rate compared to low flow rate for both studied alloys. The effects of foil composition and thickness were also investigated. In agreement with experimental results, a lower lifetime was predicted for alloy 120 than for alloy 625, with the relative difference between the two alloys increasing with increasing foil thickness.
Finally, although the \(t_{\text {10}}\) criteria were found to predict with accuracy the time to critical Cr content for the formation of the \({\hbox {Cr}_2\hbox {O}_3}\), it did not seem suitable as a lifetime criteria for a microturbine recuperator foil since the total metal loss remained limited at \(t_{\text {10}}\) for the studied alloys and experimental conditions. Therefore, a critical total metal loss was set as new lifetime criteria. The model was extended to develop a new oxidation and volatilization lifetime diagram as a function of temperature, alloy composition and oxidation and volatilization rates of the oxides after breakdown of the \({\hbox {Cr}_2\hbox {O}_3}\).
References
C. F. McDonald, Applied Thermal Engineering 23 , 1463 (2003). https://doi.org/10.1016/S1359-4311(03)00083-8
P. Maziasz, R. Swindeman, J. Shingledecker, K. More, B. Pint, E. Lara-Curzio, N. Evans, in improving high temperature performance of austenitic stainless steels for advanced microturbine recuperators, eds. U. Maney, The Institute of Materials, Minerals and Mining, Vol. Parsons 2003: Engineering Issues in Turbine Machinery, Power Plants and Renewables, (2003) pp. 1057–1073.
J. M. Rakowski, C. P. Stinner, M. Lipschutz, J. P. Montague, The use and performance of oxidation and creep-resistant stainless steels in an exhaust gas primary surface recuperator application, in: ASME Turbo Expo 2004: Power for Land, Sea and Air, Vol. 6, 103–113. (2004) https://doi.org/10.1115/GT2004-53917.
B. A. Pint, Journal of Engineering for Gas Turbines and Power 128, 370 (2006). https://doi.org/10.1115/1.2056531
C. Gindorf, L. Singheiser, K. Hilpert, Journal of Physics and Chemistry of Solids 66, 384 (2005). https://doi.org/10.1016/j.jpcs.2004.06.092
E. J. Opila, D. L. Myers, N. S. Jacobson, I. M. B. Nielsen, D. F. Johnson, J. K. Olminsky, M. D. Allendorf, Journal of Physical Chemistry A111, 1971 (2007). https://doi.org/10.1021/jp0647380.
H. Asteman, J. E. Svensson, L. G. Johansson, M. Norell, Oxidation of Metals 52, 95 (1999). https://doi.org/10.1023/a:1018875024306
G. Rother, J. R. Keiser, M. P. Brady, K. A. Unocic, L. M. Anovitz, K. C. Littrell, R. A. Peascoe-Meisner, M. L. Santella, D. J. Wesolowski, D. R. Cole, Corrosion Science 58, 121 (2012) 121–132. https://doi.org/10.1016/j.corsci.2012.01.024
M. D. Bender, R. C. Klug, Comparison of Ni-based 625 alloy and ATI 20-25+Nb\({^TM}\) stainless steel foils after long-term exposure to gas turbine engine exhaust, in: Proceedings of the ASME Turbo Expo, Vol. GT2014-25334, 2014. https://doi.org/10.1115/GT2014-25334.
J. M. Rakowski, C. P. Stinner, David S. Bergstrom, M. Lipschutz, J. P. Montague, Performance of oxidation and creep resistant alloys for primary surface recuperators for the mercury 50 gas turbine, in: Proceedings of the ASME Turbo Expo 2005, Vol. GT2005-68313, 2005, pp. 889–903.
B. A. Pint, The effect of water vapor on Cr depletion in advanced recuperator alloys, in: T. A. S. of Mechanical Engineers (ASME) (Ed.), Turbo Expo: Power for Land, Sea, and Air, Vol. Volume 1: Turbo Expo 2005, 2005, pp. 927–934.
D. J. Young, B. A. Pint, Oxidation of Metals66, 137 (2006). https://doi.org/10.1007/s11085-006-9030-1
G. R. Holcomb, Oxidation of Metals 69, 163 (2008). https://doi.org/10.1007/s11085-008-9091-4
P. Huczkowski, W. Lehnert, H. H. Angermann, A. Chyrkin, R. Pillai, D. Grüner, E. Hejrani, W. J. Quadakkers, Materials and Corrosion68, 159 (2017).
T. Sand, C. Geers, Y. Cao, J. E. Svensson, L. G. Johansson, Oxidation of Metals 92, 259 (2019). https://doi.org/10.1007/s11085-019-09935-9.
B. A. Pint, Oxidation of Metals 95, 335 (2021).
C. Tedmon, Journal of the Electrochemical Society 113, 3 (1966).
G. R. Holcomb, Journal of the Electrochemical Society 156 C292 (2009). https://doi.org/10.1149/1.3155442.
R. Pillai, S. Dryepondt, B. A. Pint, High temperature oxidation lifetime modeling of thin-walled components, in: Turbo Expo: Power for Land, Sea, and Air, Vol. ASME Turbo Expo Volume 6: Ceramics; Controls, Diagnostics, and Instrumentation; Education; Manufacturing Materials and Metallurgy, 2019, pp. 1–10.
J. L. Smialek, J. V. Auping, Oxidation of Metals 57, 559 (2002). https://doi.org/10.1023/a:1015308606869.
R. Duan, A. Jalowicka, K. Unocic, B. A. Pint, P. Huczkowski, A. Chyrkin, D. Grüner, R. Pillai, W. J. Quadakkers, Oxidation of Metals 87, 11 (2017). https://doi.org/10.1007/s11085-016-9653-9.
M. Romedenne, R. Pillai, S. Dryepondt, B. A Pint, Oxidation of Metals 96, 589 (2021). https://doi.org/10.1007/s11085-021-10069-0
A. Chyrkin, P. Huczkowski, V. Shemet, L. Singheiser, W. J. Quadakkers, Oxidation of Metals 75, 143 (2011). https://doi.org/10.1007/s11085-010-9225-3
R. Pillai, H. Ackermann, H. Hattendorf, S. Richter, Corrosion Science 75, 28 (2013). https://doi.org/10.1016/j.corsci.2013.05.013.
A. Engström, L. Höglund, J. Ågren, Metallurgical and Materials Transactions A 25, 1127 (1994). https://doi.org/10.1007/BF02652288
T. J. Nijdam, W. G. Sloof Acta Materialia56, 4972 (2008). https://doi.org/10.1016/j.actamat.2008.06.010.
R. Pillai, W. G. Sloof, A. Chyrkin, L. Singheiser, W. J. Quadakkers, Materials at High Temperatures 32, 57 (2015). https://doi.org/10.1179/0960340914z.00000000063.
R. Pillai, T. Galiullin, A. Chyrkin, W. J. Quadakkers, Calphad 53, 62 (2016). https://doi.org/10.1016/j.calphad.2016.03.004.
R. Pillai, A. Chyrkin, W. J. Quadakkers, Oxidation of Metals 96, 385 (2021). https://doi.org/10.1007/s11085-021-10033-y.
C. A. Barrett, A. Presler, COREST: A FORTRAN computer program to analyze paralinear oxidation behavior and its application to chromic oxide forming alloys, Report NASA-TN-D-8132, E-8432, NASA Lewis Research Center; Cleveland, OH, United States (1976).
MATLAB, R2019b, The MathWorks Inc., Natick, Massachusetts, (2019).
L. Kumar, R. Venkataramani, M. Sundararaman, P. Mukhopadhyay, S. P. Garg, Oxidation of Metals 45, 221 (1996). https://doi.org/10.1007/BF01046827
L. Garcia-Fresnillo, A. Chyrkin, C. Böhme, J. Barnikel, F. Schmitz, J. Quadakkers, Journal of Materials Science 49, 6127 (2014). https://doi.org/10.1007/s10853-014-8344-7
J. Garcia, O. Prat, Applied Surface Science 257, 8894 (2011). https://doi.org/10.1016/j.apsusc.2011.05.024
X. Ledoux, S. Mathieu, M. Vilasi, Y. Wouters, P. Del-Gallo, M. Wagner, Oxidation of Metals 80, 25 (2013). https://doi.org/10.1007/s11085-013-9367-1.
J. Zurek, D. Young, E. Essuman, M. Hänsel, H. J. Penkalla, L. Niewolak, W. J. Quadakkers, Materials Science and Engineering :A, 477, 259 (2008). https://doi.org/10.1016/j.msea.2007.05.035.
T. Perez, L. Latu-Romain, R. Podor, J. Lautru, Y. Parsa, S. Mathieu, M. Vilasi, Y. Wouters, Oxidation of Metals 89, 781–795 (2017).
R. Pillai, M. Romedenne, J. A. Haynes, B. Pint, Oxidation of Metals 95, 157 (2021). https://doi.org/10.1007/s11085-020-10017-4.
H. E. Evans, D. A. Hilton, R. A. Holm, S. J. Webster, Oxidation of Metals 14, 235 . https://doi.org/10.1007/BF00604566.
H. E. Evans, A. T. Donaldson, T. C. Gilmour, Oxidation of Metals 52, 379 (1999). https://doi.org/10.1023/A:1018855914737
J. E. Croll, G. R. Wallwork, Oxidation of Metals 1, 55 (1969). https://doi.org/10.1007/BF00609924.
J. Chase, M.W., NIST-JANAF Themochemical Tables, eds. M.Fourth Edition, J. Phys. Chem. Ref. Data (Ed.), NIST-JANAF Themochemical Tables, second edition Edition, 1998, pp. 1–1591.
N. Provatas, K. Elder, Materials Science and Engineering (2010). https://doi.org/10.1002/9783527631520.
T. Alberto, A. Massardo, Applied Thermal Engineering 25, 2054 . https://doi.org/10.1016/j.applthermaleng.2005.01.015.
R. Pillai, B. A. Pint, JOM 73, 3988 (2021). https://doi.org/10.1007/s11837-021-04972-9.
Capstone develops hydrogen microturbine. https://www.turbomachinerymag.com/view/capstone-develops-hydrogen-microturbine
Acknowledgements
The author would like to thank G. W. Garner, M. S. Stephens, J. Wade, T. Lowe, V. Cox at ORNL for their assistance with the experimental work and P. F. Tortorelli and A. Haynes for their valuable comments on the manuscript. This research was sponsored by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Combined Heat and Power Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Rights and permissions
About this article
Cite this article
Romedenne, M., Pillai, R., Dryepondt, S. et al. Oxidation Lifetime Modeling of 625 and 120 Foils After Long-term Exposure in Flowing Air + 10% \({\hbox {H}_2\hbox {O}}\) at 700 and 800 \(^\circ\)C. Oxid Met 98, 305–324 (2022). https://doi.org/10.1007/s11085-022-10124-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-022-10124-4