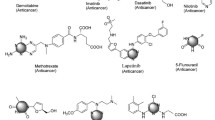
Cancer is a rapid, out of control, and proliferation pathology of ordinary cells, which has become one of the most infamous and aggressive health afflictions worldwide. Molecular hybridization is related to a combination of two or more pharmacophores of bioactive frames which generate a single molecular structure with enhanced activity. Previous studies described the piperazine framework as a central structure in numerous synthetic and natural compounds, showing a wide range of biological activities. Here, we review, highlight and discuss a detailed account of the anticancer applications of some important piperazine-containing hybrid heterocycles; we have also included several patents approved for the anticancer activity of piperazine heterocycles.
Similar content being viewed by others
INTRODUCTION
Due to rapid spreading, the cancer cells in any part of the body, without control of tumor growth cause increase in cancer cases in the world, whereby cancer related diseases could very well be one of the most perilous health hazards. Therefore, it is fundamental to search for various effective and moderate cure molecules for this fatal disease, which in fact ranks as one of the foremost reasons of death around the world. On a positive note, piperazine is a typical heterocyclic compound considered as one of the most significant, critical, and essential building frames of many important natural and synthetic anticancer compounds [1,2,3,4,5,6,7,8,9,10, – 11]. The piperazine moiety demonstrates its achievement in numerous compounds as the fundamental focus for many anticancer agents and this success is definitely supported by approving the 2-oxopyrazine (Olaparib) and 2-aminopyrimidine (Imatinib, Abemaciclib, Palbociclib and Rociletinib) derivatives (Fig. 1) by the FDA as a targeted therapy for treating cancer. LYG-202 has also been confirmed as possessing an antitumor effect, however, the mechanism is still ambiguous. The introduction of bioactive groups into piperazine has profound potential application in medicinal chemistry. Piperazine cores combined with various heterocyclic scaffolds offers a new class of hybrid heterocycles displaying profound activity. The biological applications of piperazine derivatives and their attached heterocyclic derivatives have drawn research interest in recent years because of their wide range of biological activities including antimicrobial [12], antifungal [13], anti-inflammatory [14], and anticancer [15, 16].
We have observed new and modified heterocyclic analogs to piperazine endowed with high potency toward the anti-proliferative activity. This review focuses on the antiproliferative activity of piperazine-heterocyclic hybrids, which has accounted for in vitro cell growth inhibitory effect in various tumor cell lines. The incorporation of piperazine scaffold into five heterocyclic rings including thiazole, pyrrole, pyrazole and triazole was found a potent inducer of differentiation in anti-proliferative activity.
PIPERAZINE HETEROCYCLE BASED ANTICANCER DERIVATIVES
Piperazine derivatives bearing various heterocyclic scaffolds are interesting pharmacophores because of their wide spectrum of properties. Here, we focus on the anticancer activity of several piperazine bearing six- and five-member heterocyclic rings.
El-Damasy, et al. [17] have reported on various picolinamide derivatives, whereby the analogs demonstrated the anti-proliferative activity against most of cancer cell lines. Compound 1 with piperazine containing substituted thiazole exhibited high impact on native (IC50 = 18.2 μM) and T315I mutant ABL (IC50 = 39.9 μM) and displayed maximum activity with growth inhibition (89.8%) against K-562 cell line.

In our previous work [18], benzothiazole moiety was incorporated into C4-position of ethyl 2-(piperazin-1-yl) acetate and the anticancer activity of benzothiazole moiety was tested against Dalton’s lymphoma ascites cells. Compound 2 with bromo group exhibited excellent anticancer activity, whereby the tumor regression reduced excretion of the ascites up to three-fold compared to cells.

Among the numerous amino-substituted benzimidazole analogs evaluated against the human breast cancer cells using 2D and 3D assays, derivative 3 bearing piperazine in any positions of phenyl and piperidine showed selective activity on MDA-MB-23 in 3D cell culture system [19].

Indolin-2-one scaffold modified by attached 4-phenyl piperazine-1-carbothiohydrazides at the C3-position, and anti-proliferative activity was tested against three human cancer cell lines. Compounds 4b and 4c prevented A549 cell growth at IC50 values 3.59 and 5.58 μM, respectively, whereas compounds 4a and 4c showed good activity versus HCT-116, similar to the standard drug Sunitinib, with IC50 values 3.49 and 4.57 μM [20].

New hybridization between saccharin and arylpiperazine was reported by Chen [21]. The new compounds were evaluated as anticancer against LNCaP, DU145 and PC-3 cancer cell lines. According to the results of cellular assays, it was found that this group of compounds displayed significant activity. Compounds 5a and 5b bearing phenyl and p-fluoro phenyl showed high cytotoxic activity against DU145 cells (IC50 < 2 μM).

Akkoc [22] presented piperazine related indole as an anticancer agent [22] and the test results showed good to moderate anti-cancer activity against HUH7, MCF7 and HCT116 human tumor cell lines. Attractive feature of the research was the introduction of 3,4-dichlorophenyl onto piperazine moiety (6) which proved a key fragment for increasing the potency compared to the standard drug (5-FU) with IC50 values of 2.92, 3.42, and 9.33 μM.

Murty et al [23] developed piperazine linked phenyl triazole derivatives which had considerable cytotoxicity against numerous cancer cell lines. The chloro substituent in positions 3 or 4 of benzene increased the selectivity so that compounds 7a and 7b proved to be best potent agents against HL-60 and U937cells with IC50 within 6.67 – 52.33 μM.

In 2016, Jiang, et al. [24] developed substituted piperazine-indole hybrids [24]. Compounds 8a and 8b exhibited highly potent anticancer activities against Hela (IC50 4.06 μM) and ECA-109 (IC50 2.13 μM) cells.

In vitro anticancer activity of new piperazinyl imidazoles have been evaluated against MCF-7, HEK 293 and HeLa cancer cell lines at different concentrations. Compound 9 was the most potent and selective, exhibiting an incredibly strong activity against MCF-7 and HeLa cells at IC50 14.05 and 17.64, respectively [25]. A molecular docking study was used for supporting the anticancer activity relationship against quinone reductase-2 protein using Doxorubicin as standard drug. The docking result revealed good interactions of hydrogen bond with various amino acids.

New N-aryl piperazine-benzofuran derivatives were designed, prepared and examined for their antitumor properties against various human cell lines using MTT assay. According to results, compound 10 exhibited the best activity against four strains of cell lines and displayed selective cytotoxic activity against Hela with IC50 = 0.03 μM [26].

Several purine ribonucleoside derivatives with piperazine introduced into 4-position exhibited emerging good antitumor activity against MCF7, HepG2, FOCUS, Huh7 and HCT116 carcinoma cell lines. Derivative 11 was the best anticancer candidate with IC50 values 5.2 and 9.2 μM [27], which also influenced cellular kinase activity through interference with cellular ATP reserves. In addition, compound 11 induced senescence-associated cell death as revealed by SAβ-gal assay.
When substituted piperazine was conjugated with piperidine, pyridazine, pyrimidine, thiazine and triazine, the anticancer activity was significantly enhanced. Fytas, et al. [28] developed various adamantly-piperazine analogs and investigated their anti-proliferative effect against MDA MB 231, HeLa, NCI H1975 and MIA PaCa2 cancer cells. Substituted piperazine 12 was most potent against MDA MB 231 and HeLa tumor cell lines with IC50 = 8.4 and 9.2 μM, respectively. Compound 12 showed low cytotoxicity level against the NHDF and HUVEC human cells.

The new synthetic analogs of 1,4-substituted piperazines bearing pyridazines were evaluated for their anticancer activity against SKBR3, HeLa, HCT116, H1299 and A375 cancer cell lines. HCT116 and HeLa were highly sensitive with respect to the examined compounds. Compound 13 displayed good activity [29].

The anti-proliferative activities of synthesized piperazine-quinazoline derivatives were also evaluated against MCF-7, A549, HT29, HeLa and HCT-116 human cancer cell lines. Compounds 14a, 14b and 14c were the most promising and active ones [30]. They also displayed from weak to no activity related to dihydrofolate reductase and thymidylate

Several benzothiazole-piperazine derivatives were prepared with various functional groups at the N-terminal of piperazine and investigated for their antitumor activity against MIAPACA, HeLa, MDA-IMR32 and MB-231 cells. Among these, derivatives 15a and 15b displayed interesting growth inhibitory effects, especially in neuroblastoma cell lines and breast cancer cell lines. Compound 15a showed high potent cytotoxicity with GI50 values in the range of <10 μM. while compound 15b exhibited good growth inhibition against neuroblastoma and breast cell lines with GI50 values of 0.12, 0.63 and 0.83 μM [31].

Yurttaş et al. [32] developed piperazinyl 1,2,4-triazine derivatives and evaluated their antitumor activity against MCF-7 using BrdU, XTT and NIH/3T3 methods. Among the tested drugs, compounds 16a and 16b were found to have potential anti-proliferative activity in comparison with cisplatin as an anti-cancer drug.
In 2017, Bu, et al. [33] described the synthesis of new ergosterol peroxide 3-carbamate derivatives carrying the piperazine skeleton and tested them for antitumor activity against SK-Hep1, HepG2 and MDA-MB231, and MCF-7 cells. Compound 17 displayed significant anti-proliferative activity against these cell lines, with IC50 values from 0.85 to 4.62 μM.
Shallal et al, [34] described combining piperazine and pyrimidine moieties in a single framework to analyzed its anticancer activity. Substituents 18a and 18b exhibited high inhibition of MDA-MB-468, a triple negative/basal-like breast carcinoma cell line.

Zhang, et al. [35] synthesized new derivatives of substituted piperazin-quinazoline and studied their anti-proliferative activity. Compound 19 showed promising anti-proliferative activity against A375, PC3, A549 and MGC803 cells with IC50 values of 1.8, 1.3, 2.9 and 2.8 μM, respectively, which demonstrated better inhibition than that of gefitinib (7.2, 7.2, 9.8 and 7.6, μM, respectively). At the same time, compound 19 showed very low IC50 values against NH3T3 cells.
Multicomponent molecules with at least three components in the same structure offer good approach to development of bioactive compounds. Substituted 5,8-dihydropteridine-6,7-dione of piperazine was evaluated for its anti-proliferative activity against SGC-7901, A549, MGC-803 and PC-3 cancer cell lines. Among the drugs tested, compound 20 exhibited the most potent anti-proliferative activity and showed slightly higher activity than 5-FU against the MGC-803 cell line and inhibited the formation of colonies and MGC-803 cells migration [36].

The anti-proliferative potential of piperazine containing benzothiazole and triazole derivatives was evaluated against MCF7, HCT116, Caco2 and T47D human cancer cells growth by MTT assay. Compound 21 showed the most pronounced activity against T47D, MCF7, HCT116 and Caco2 carcinoma cell lines with IC50 values of 38, 33, 48 and 42 μM. respectively [37].

Sun, et al. [38] studied the molecular hybridization synthesis of podophyllotoxin-piperazine derivatives and studied their anti- proliferation activity on some human cancer cell lines. Compounds 22 indicated potent cytotoxicity against cancer cells, with specifically high activity against MCF-7 cell lines with an IC50 = 2.78 ± 0.15 μM.
Recently, Li, et al. [39] discussed the synthesis and anti-proliferative activities of pteridine derivatives against MGC-803, MKN-45, H1650 and EC-109. Among the congeners, 23 bearing piperazine ring displayed the highest potency of anti-proliferative activity against MGC-803 and in induced
apoptosis of MKN-45 with the IC50 values of 7.01 and 4.32 μM, respectively [39].

Several piperazinyl-linked palbociclib analogs were designed and evaluated for their anti-proliferation activity against MDA-MB-231 and MDA-MB-453 cell lines. Two of the examined compounds (24a and 24b) exhibited considerable anticancer activities, whereby 24a was observed to have better cytotoxicity than the palbociclib standard drug [40].

Recent experiments focused on the anti-proliferative activity of piperazine derivatives bearing quinazoline moiety were reported in 2016 [41], according to which most of these compounds inhibited activity against A549, MCF-7 and HCT-116 cell lines. Compound 25 was selected as a typical anti-proliferative drug with IC50 values around 10 M [41].

A series of new hybrid imidazo[1,2-a]pyrimidine-piperazine analogs were prepared and examined for their potential antitumor activity. Molecules 26 and 27 exhibited potent effectiveness with GI50 values similar to that of doxorubicin [42].

The anti-proliferative effects of new thiazolidinones bearing piperazine analogs were evaluated on human leukemia cells. Compound 28 exhibited strong activity against Jurkat, Nalm6, and K562 cells (IC50 15.24, 19.29 and 9.71 μM, respectively). In addition, it induced cell cycle arrest and caused depolarization of the mitochondrial membrane potential [43].
Triazole-piperazine-phenanthridine derivatives were synthesized by the cycloaddition method for azide-alkyne using copper(I) catalyst. Compound 29 was the best anti-proliferative agent in comparison to reference drug, with IC50 = 7.22 ± 0.32 μM against HL60 cell line [44].

A series of piperazinyl-1,3,4-oxadiazole compounds were designed and their anticancer activity was tested against Hela, HepG2, BGC823, SW1116, HepG2, Hela, SW116 and BGC823 cell lines. Compound 30 was the most potent anti-proliferative agent against HepG2 and SW1116 cells with IC50 = 5.78 and 47.15 μM, respectively, and showed maximum anti-FAK activity with IC50 = 0.78 μM [45].

The indoloquinoline-piperazine complex was prepared by combining the indoloquinoline backbone with piperazine and their ruthenium- and osmium-arenes, after which they were examined for their in vitro anticancer activity against (NCI)-60 cell line. Compounds 31a and 31b demonstrated significance of the metal-binding position for their cytotoxicity and showed most promising anti-proliferative activity [46].

In vitro anticancer activity of new piperazine analogs was assessed against DU-145. Compound 32 displayed antiproliferative activity at IC50 of 14.1 μg/mL, showed strong inhibition of DU-145 cell growth, and was more efficient than the standard drug ADR [47].

A series of piperazinyl-quinoline hybrids were synthesized and tested for their in vitro anticancer activity against two cell lines (SK-N-SH and A549). Among the group tested, compound 33 was reported to have the most potent anticancer activity (IC50 = 16.2) [48].

A series of new 4-(1H-indole-2-carbonyl)piperazine-2,6-diones were prepared by combining piperazine-2,6-dione derivatives with indole carboxylic acid under microwave irradiation, and then tested for their anticancer activity against HCT-15, NCI H-522, T47D, HepG-2 and PA-1 cell lines at a concentration of 10 μM. Compounds 34a, 34b, and c showed good anticancer activity [49].

New piperazine analogs were investigated for their antiproliferative activity against PC-3 and MCF-7 cell lines. Substituted piperazine 35 exhibited significant cytotoxicity against both cell lines at IC50 values of 6.50 and 11.75 μM, respectively, while the IC50 values of standard drug doxorubicin were 6.77 μM (MCF-7) and 7.73 μM (PC3) [50].

New classes of triazole linking to piperazine scaffolds were reported as anticancer agents. For example, compound 36 showed potent anticancer activity comparable to that of oxorubicin and induced apoptosis in Cal 72 cells by targeting mitochondrial pathways with an IC50 value of 1.92 μM [51].

El-Miligy, et al. [52] synthesized a series of new piperazine based thiazolidinones and evaluated them as potential anticancer drugs. Compounds 37a, 37b and 37c showed good anticancer activity with IC50 values 0.03 – 0.06 μM against HepG-2. They also induced caspase-dependent apoptosis in HepG-2 cell line.
In conclusion, piperazine bearing heterocycles proved to be an excellent platform in the field of medicinal chemistry and showed various applications as anticancer agents. Remarkable results obtained for these compounds helped and stimulated researchers to synthesize new piperazine derivatives. The resent review described piperazine containing heterocycles and showed evidence of their great potential as anticancer agents. As reported, cell growth inhibition by piperazine derivatives was demonstrated in vitro on a variety of tumor cell lines.
References
P. Meena, V. Nemaysh, M. Khatri, et al., Bioorg. Med. Chem., 23(5), 1135 – 1148 (2015).
R. V. Patel, B. Mistry, R. Syed, et al., Eur. J. Pharm. Sci., 88, 166 – 177 (2016).
K. H. Rohde, H. A. Michaels, A. Nefzi, et al., Bioorg. Med. Chem. Lett., 9, 2206 – 2209 (2016).
M. Taha, M. Irshad, S. Imran, et al., Eur. J. Med. Chem., 141, 530 – 537 (2017).
M. Aggarwal, R. Kaur, A. Saha, et al., Antivir. Res., 146, 102 – 111 (2017).
M. Çeçen, J. M. Oh, Z. Özdemir, et al., Molecules, 22, 5371 (2020).
Y. Xu, P. Liang, H. Ur Rashid, et al., Med. Chem. Res., 10, 1618 – 1627 (2019).
B. N. Saðlýk, O. Cebeci, U. Acar Çevik, et al., Molecules, 18, 4342 – 4347 (2020).
G. Y. Zhang, Z. Zhang, K. Li, et al., Bioorg. Chem., 105, 104398 (2020).
Y. Chu, B. R. S. Reddy, V. P. R. Gajulapalli, et al., Bioorg. Med. Chem. Let., 24, 127613 (2020).
W. Li, S. Y. Chen, W. N. Hu, et al., J. Chem. Res., 9, 536 – 542 (2020).
K. S. Thriveni, B. Padmashali, M. B. Siddesh, et al., Ind. J. Pharm. Sci., 4, 332 – 338 (2014).
S. Radi, Y. Toubi, A. Hakkou, et al., Lett. Drug. Des. Discovery, 9, 853 – 857 (2012).
D. C. Batista, D. P. B. Silva, I. F. Florentino, et al., Inflammopharmacology, 26, 217 – 226 (2018).
E. E. Gurdal, E. Buclulgan, I. Durmaz, et al., Anticancer Agents Med. Chem., 15, 382 – 389 (2015).
N. Inceler, A. Yilmaz, S. N. Baytas, et al., Med. Chem. Res., 22, 3109 – 3118 (2013).
A. K. El-Damasy, S. H. Seo, N. C. Cho, et al., Eur. J. Med. Chem., 101, 754 – 768 (2013).
M. Al-Ghorbani, G. S. Pavankumar, P. Naveen, et al., Bioorg. Chem., 65, 110 – 117 (2016).
N. Perin, K. Bobanoviæ, I. Zlatar, Eur. J. Med. Chem., 125, 722 – 735 (2017).
H. H. Lin, W. Y. Wu, S. L. Cao, et al., Bioorg. Med. Chem. Lett., 23 3304 – 3307 (2013).
H. Chen, B. B. Xu, T. Sun, et al., Molecules, 22, 1857 – 1866 (2017).
M. K. Akkoç, M. Y. YükseL, Ý. Durmaz, et al., Turk. J. Chem., 4, 515 – 525 (2012).
M. S. R. Murty, M. R. Katiki, B. R. Rao, et al., Lett. Drug Des. Discovery, 9, 968 – 981 (2016).
J. R. Jiang, F. Xu, H. G. Wu, et al., J. Chem., 1, 2016 (2016).
L. Boddu, A. K. Pagudala, D. Gandamalla, et al., J. Mol. Struct., 1166, 362 – 368 (2018).
Z. Mao, X. Zheng, Y. Lina, et al., Bioorg. Med. Chem. Lett., 15, 3421 – 3424 (2016).
M. Tuncbilek, E. B. Guven, T. Onder, et al., J. Med. Chem., 55, 3058 – 3065 (2012).
C. Fytas, G. Zoidis, A. Tsotinis, et al., Eur. J. Med. Chem., 26, 281 – 290 (2015).
M. S. Murty, B. Ramalingeswara Rao, K. R. Ram, et al., Med. Chem. Res., 21, 3161 – 3169 (2012).
S. L. Cao, Y. Han, C. Z. Yuan, et al., Eur. J. Med. Chem., 64, 401 – 409 (2013).
R. Venkatesh, S. Kasaboina, D. Bidayat, et al., Bioorg. Med. Chem. Lett., 27, 354 – 359 (2017).
L. Yurttaş, S. Demirayak, S. Ilgýn, et al., Bioorg. Med. Chem., 22, 6313 – 6323 (2014).
M. Bu, T. Cao, H. Li, et al., Chem. Med. Chem., 6, 466 – 474 (2017).
H. M. Shallal, W. A. Russu, et al., Eur. J. Med. Chem., 46, 2043 – 2057 (2011).
Y. Zhang, Y. Huang, H. Xiang, et al., Eur. J. Med. Chem., 78, 23 – 34 (2014).
P. F. Geng, C. C. Wang, Z. H. Li, et al., Eur. J. Med. Chem., 143, 1959 – 1967 (2018).
S. B. Ozdemir, Y. U. Cebeci, H. Bayrak, et al., Heterocycl. Commun., 23, 43 – 54 (2017).
W. X. Sun, Y. J. Ji, Y.Wan, et al., Bioorg. Med. Chem. Lett., 27, 4066 – 4074 (2017).
Z. H. Li, T. Q. Zhao, X. Q. Liu, et al., Eur. J. Med. Chem., 1396 – 1405 (2018).
P. Wang, J. Huang, K. Wang, et al., Eur. J. Med. Chem., 122, 546 – 556 (2016).
Y. Zhang, C. R. Yang, X. Tang, et al., Bioorg. Med. Chem. Let., 19, 4666 – 4670 (2016).
R. Aeluri, M. Alla, S. Polepalli, N. Jain, et al., Eur. J. Med. Chem., 100, 18 – 23 (2015).
K. S. Kumar, A. Hanumappa, M. Hegde, et al., Eur. J. Med. Chem., 81, 341 – 349 (2014).
H. N. Nagesh, N. Suresh, G. V. Prakash, et al., Med. Chem. Res., 24, 523 – 532 (2015).
J. Sun, S. Z. Ren, X. Y. Lu, et al., Bioorg. Med. Chem., 25, 2593 – 2600 (2017).
L. K. Filak, D. S. Kalinowski, T. J. Bauer, et al., Inorg. Chem., 53, 6934 – 6943 (2014).
R. V. Patel, P. K. Patel, P. Kumari, et al., Eur. J. Med. Chem., 53, 41 – 51 (2011).
L. Nagarapu, H. K. Gaikwad, R. Bantu, et al., Eur. J. Med. Chem., 46, 2152 – 2156 (2011).
S. Kumar, N. Kumar, P. Roy, et al., Med. Chem. Res, 22, 4600 – 4609 (2013).
M. N. Aboul-Enein, A. M. El-Azzouny, F. A. Ragab, et al., Arch. Pharm. Chem. Life Sci., 3, 350 (2017).
C. B. Mishra, R. K. Mongre, S. Kumari, et al., ACS Chem. Biol.,12, 753 – 768 (2017).
M. El-Miligy, H. A. Abd El Razik, M. M. Abu-Serie, et al., Future Med. Chem., 9, 1709 – 1729 (2017).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Ghorbani, M., Gouda, M.A., Baashen, M. et al. Piperazine Heterocycles as Potential Anticancer Agents: A Review. Pharm Chem J 56, 29–37 (2022). https://doi.org/10.1007/s11094-022-02597-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02597-z