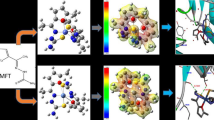
In the present work, Morita—Baylis–Hillman (MBH) adduct ligand 3 was prepared under MBH reaction conditions. The compound was purified by chromatographic techniques and structurally confirmed by methods of spectral analysis. Ligand 3 was separately treated with various salts of transition metals (Mn, Cu, Ce and Lu) under basic conditions with and reflux and stirring. This treatment yielded complexes 5–8, the molecular and structural formulas of which were established by spectral and elemental analysis. Then, complexes 5–8 were checked for their antibacterial potential against four strains (E. coli, S. aureus, P. mirabilis, and P. aeruginosa). Almost all compounds exhibited good activity except Mn and Lu complexes which were inactive against S. aureus and P. aeruginosa, respectively. Cerium complex showed moderate antibacterial activity against all selected strains. Among all, Cu complex was fund to be the most active one against the selected bacterial strains. This study demonstrated the first example of using the MBH adduct as ligand for the formation of complexes.



Similar content being viewed by others
References
P. H. Paioti, and F. Coelho, Tetrahedron Lett., 52(46), 6180 – 6184 (2011).
S. R. Reddy, S. T. Das, and T. Punniyamurthy, Tetrahedron Lett., 45(18), 3561 – 3564 (2004).
Y. Zheng, X. Li, C. Ren, et al., J. Org. Chem., 77(22), 10353 – 10361 (2012).
S. Bhowmik and S. Batra, Curr. Org. Chem., 18(24), 3078 – 3119 (2014).
H. H. Kuan, R. J. Reddy, and K. Chen, Tetrahedron, 66(52), 9875 – 9879 (2010).
A. K. Pandey, S. H. Han, N. K. Mishra, et al., ACS Catalysis, 8(1), 742 – 746 (2017).
N. M. Aljamali and N. S. Salih, Int. Technol. Innov. Res. J., 1(1), 1 – 8 (2015).
S. K. Arupula, S. Guin, A. Yadav, et al., J. Org. Chem., 83(5), 2660 – 2675 (2018).
W. Amaranth, M. Benassi, R. N. Pascoal, et al., Tetrahedron, 66(24), 4370 – 4376 (2010).
H. Elleuch, W. Mihoubi, M. Mihoubi, et al., Bioorg. Chem., 78, 24 – 28 (2018).
J. Lasri and A. M. J. Charmier, J. Org. Chem., 72(21), 7550 – 7555 (2007).
C. G. Lima-Junior and M. L. Vasconcellos, Bioorg. Med. Chem., 20(13), 3954 – 3971 (2012).
J. K. Ekegren, P. Roth, K. Källström, et al., Org. Biomol. Chem., 1(2), 358 – 366 (2003).
J. Devi and N. Batra, Spectrochim. Acta Part A: Mol. Biomol. Spectrosc., 135, 710 – 719 (2015).
E. G. Deze, S. K. Papageorgiou, E. P. Favvas, and F. K. Katsaros, Chem. Eng. J., 209, 537 – 546 (2012).
S. R. Annapure, R. A. Waghmare, S. D. Rathod, and N. P. Adlinge, Asian J. Res. Chem., 10(4), 541 – 545 (2017).
N. R. Barboza, M. M. Morais, P. S. Queiroz, et al., Frontiers in Microbiol., 8, 1946 (2017).
T. Mangamamba, M. C. Ganorkar, G. Swarnabala, Int. J. Inorg. Chem., 2014, 1 (2014).
A. Rodrìguez, J. A. Garcìa-Vázquez, A. Sousa-Pedrares, et al., Inorg. Chem. Commun., 6, 619 – 622 (2013).
H. Ullah, A. V. Ferreira, J. A. Bendassolli, et al., Synthesis, 47(1), 113 – 123 (2015).
E. Labisbal, K. D. Haslow, A. Sousa-Pedrares, et al., Polyhedron, 22(20), 2831 (2003).
A. N. M. Alaghaz, H. A. Bayoumi, Y. A. Ammar, and S. J. Aldhlmani, Mol. Struct., 1035, 383 – 399 (2013).
N. Busto, J. Valladolid, C. Aliende, et al., Chem. Asian J., 7(4), 788 – 801 (2012).
S. Y. Liu, I. D. Hills, and G. C. Fu, Organometallics, 21(21), 4323 – 4325 (2002).
C. U. Aguoru and G. Audu, Greener J. Biol. Sci., 2(2), 28 (2012).
H. N. Aliyu and U. Sani, Bayero J. Pure Appl. Sci., 4(1), 83 – 87 (2011).
N. Ahmad, B. H. Abbasi, and H. Fazal, Toxicol. Ind. Health, 32(3), 500 – 506 (2016).
H. Fazal and A. Rauf, Pak. J. Pharm. Sci., 28(6), 2091 – 2094 (2015).
H. Fazal, N. Ahmad, B. H. Abbasi, and N. Abbass, Pak. J. Bot., 44(3), 1103 – 1109 (2012).
M. Ikram, G. Jan, S. Dad, et al., Int. J. Phytomed., 5(4), 475 – 478 (2013).
K. B. Rabia, M. Sultan, and H. Zakir, Am. J. Pharm. Tech. Res., 3, 283 (2013).
H. D. Revanasiddappa, H. D. Vijaya, S. L. Kumar, and K. L. Parasad, World J. Chem., 5(1), 18 – 25 (2010).
M. N. Khan, H. Ullah, S. Hussain, and A. K. Khattak, Pak. J. Pharm. Sci., 31(1), 103 (2018).
S. Nzikayel, I. J. Akpan, and E. C. Adams, Med. Chem. (Los Angeles), 8, 26 – 28 (2018).
A. Sugino, C. L. Peebles, K. N. Kreuzer, and N. R. Cozzarelli, Proc. Natl. Acad. Sci. USA, 74(11), 4767 – 4771 (1977).
ACKNOWLEDGMENTS
The authors are thankful to MIU AJK for providing infrastructure and also thankful to PCSIR-Peshawar, Pakistan, for support in carrying out elemental analysis, spectral analysis and bioactivity studies.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ishfaq, S., Ullah, H., Rahman, T.U. et al. Morita Baylis Hillman Adduct Serves as Ligand in the Synthesis of Transition Metal Complexes Exhibiting Antibacterial Activity. Pharm Chem J 56, 906–912 (2022). https://doi.org/10.1007/s11094-022-02725-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-022-02725-9