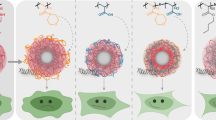
Abstract
Purpose
To optimize oligonucleotide (ODN)-based polyion complex micelles (PICMs) by studying the effects of polymer composition and length on their properties.
Methods
Atom transfer radical polymerization was used to synthesize copolymers with increasing hydrophilic nonionic and cationic block lengths. PICMs were prepared by mixing the copolymers and ODN at various nitrogen-to-phosphate (N/P) ratios and characterized by gel electrophoresis and dynamic light scattering. The stability of the complexes towards dissociation was tested using a competitive assay with heparin. Finally, protection of the incorporated ODN against DNAse I degradation was evaluated.
Results
A library of copolymers composed of poly(ethylene glycol) (PEG) and poly(aminoethyl methacrylate) (PAEMA) and/or poly((dimethylamino)ethylmethacrylate) (PDMAEMA) was synthesized. All polymers efficiently interacted with the ODN at N/P ratios approaching 1.5. Narrowly distributed but easily dissociable PICMs were obtained using PEG 5000 and short DMAEMA chains. Shortening the PEG block to 2000, increasing the number of cationic units and using AEMA produced more stable complexes but at the cost of colloidal properties. All polymers were able to protect the ODN from nuclease degradation.
Conclusions
PEG 3000-based PICMs possess good colloidal properties, intermediate stability towards dissociation and adjustable buffering capacity, making them potentially useful for the delivery of nucleic acid drugs.






Similar content being viewed by others
Abbreviations
- AEMA:
-
2-aminoethyl methacrylate
- AEMABoc:
-
2-(N-(tert-butoxycarbonyl)amino)ethyl methacrylate
- ATRP:
-
atom transfer radical polymerization
- β :
-
buffering capacity
- DLS:
-
dynamic light scattering
- DMAEMA:
-
2-(N,N-dimethylamino)ethyl methacrylate
- EDTA:
-
ethylenediaminetetraacetic acid
- EO:
-
ethylene oxide
- EtBr:
-
ethidium bromide
- GPC:
-
gel permeation chromatography
- 1H NMR:
-
proton nuclear magnetic resonance
- M n :
-
number-average molecular weight
- M w :
-
weight-average molecular weight
- MW:
-
molecular weight
- N/P:
-
nitrogen-to-phosphate
- ODN:
-
oligodeoxyribonucleotide
- PEG:
-
poly(ethylene glycol)
- PEI:
-
poly(ethyleneimine)
- PI:
-
polydispersity index
- PICMs:
-
polyion complex micelles
- siRNA:
-
small interfering RNA
- SD:
-
standard deviation
- THF:
-
tetrahydrofuran
References
B. A. Bunnell, and R. A. Morgan. Gene therapy for infectious diseases. Clin. Microbiol. Rev. 11:42–56 (1998).
I. A. McNeish, S. J. Bell, and N. R. Lemoine. Gene therapy progress and prospects: cancer gene therapy using tumour suppressor genes. Gene Ther. 11:497–503 (2004).
J. C. T. van Deutekom, and G. J. B. van Ommen. Advances in Duchenne muscular dystrophy gene therapy. Nat. Rev. Genet. 4:774–783 (2003).
S. Ferrari, D. M. Geddes, and E. W. F. W. Alton. Barriers to and new approaches for gene therapy and gene delivery in cystic fibrosis. Adv. Drug Deliv. Rev. 54:1373–1393 (2002).
S. Agrawal, J. Temsamani, and J.Y. Tang. Pharmacokinetics, biodistribution, and stability of oligodeoxynucleotide phosphorothioates in mice. Proc. Natl. Acad. Sci. U. S. A. 88:7595–7599 (1991).
E. Wickstrom. Oligodeoxynucleotide stability in subcellular extracts and culture media. J. Biochem. Biophys. Methods. 13:97–102 (1986).
A. Rifai, W. Brysch, K. Fadden, J. Clark, and K. H. Schlingensiepen. Clearance kinetics, biodistribution, and organ saturability of phosphorothioate oligodeoxynucleotides in mice. Am. J. Pathol. 149:717–725 (1996).
M. D. Hughes, M. Husssain, Q. Nawaz, P. Sayyed, and S. Akhtar. The cellular delivery of antisense oligonucleotides and ribozymes. Drug Discov. Today. 6:303–315 (2001).
J. Kurreck. Antisense tchnologies. Improvement through novel chemical modifications. Eur. J. Biochem. 270:1628–1644 (2003).
S. C. De Smedt, J. Demeester, and W. E. Hennink. Cationic polymer based gene delivery systems. Pharm. Res. 17:113–126 (2000).
E. Mastrobattista, M. A. E. M. van der Aa, W. E. Hennink, and D. J. A. Crommelin. Artificial viruses: a nanotechnological approach to gene delivery. Nat. Rev. Drug Discov. 5:115–121 (2006).
D. W. Pack, A. S. Hoffman, S. Pun, and P. S. Stayton. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 4:581–593 (2005).
D. Putnam. Polymers for gene delivery across length scales. Nat. Mater. 5:439–451 (2006).
A. V. Kabanov, S. V. Vinogradov, Y. G. Suzdaltseva, and V. Y. Alakhov. Water-soluble block polycations as carriers for oligonucleotide delivery. Bioconjugate Chem. 6:639–643 (1995).
K. Kataoka, H. Togawa, A. Harada, K. Yasugi, T. Matsumoto, and S. Katayose. Spontaneous formation of polyion complexe micelles with narrow distribution from antisense oligonucleotide and cationic block copolymer in physiological saline. Macromolecules. 29:8556–8557 (1996).
C. Plank, K. Mechtler, F. C. Szoka, and E. Wagner. Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum. Gene Ther. 7:1437–1446 (1996).
M. Ogris, S. Brunner, S. Schuller, R. Kircheis, and E. Wagner. PEGylated DNA/transferrin–PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 6:595–605 (1999).
H. Petersen, P. M. Fechner, A. L. Martin, K. Kunath, S. Stolnik, C. J. Roberts, D. Fischer, M. C. Davies, and T. Kissel. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjugate Chem. 13:845–854 (2002).
C. Brus, H. Petersen, A. Aigner, F. Czubayko, and T. Kissel. Physicochemical and biological characterization of polyethylenimine-graft-poly(ethylene glycol) block copolymers as a delivery system for oligonucleotides and ribozymes. Bioconjugate Chem. 15:677–684 (2004).
S. Mao, M. Neu, O. Germershaus, O. Merkel, J. Sitterberg, U. Bakowsky, and T. Kissel. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/siRNA polyplexes. Bioconjugate Chem. 17:1209–1218 (2006).
S. Sundaram, L. K. Lee, and C. M. Roth. Interplay of polyethyleneimine molecular weight and oligonucleotide backbone chemistry in the dynamics of antisense activity. Nucleic Acids Res. 35:4396–4408 (2007).
S. Sundaram, S. Viriyayuthakorn, and C. M. Roth. Oligonucleotide structure influences the interactions between cationic polymers and oligonucleotides. Biomacromolecules. 6:2961–2968 (2005).
E. Ramsay, and M. Gumbleton. Polylysine and polyornithine gene transfer complexes: A study of complex stability and cellular uptake as a basis for their differential in-vitro transfection efficiency. J. Drug Target. 10:1–9 (2002).
P. van de Wetering, E. E. Moret, N. M. E. Schuurmans-Nieuwenbroek, M. J. van Steenbergen, and W.E. Hennink. Structure–activity relationships of water-soluble cationic methacrylate/methacrylamide polymers for nonviral gene delivery. Bioconjugate Chem. 10:589–597 (1999).
M. A. Wolfert, P. R. Dash, O. Nazarova, D. Oupicky, L. W. Seymour, S. Smart, J. Strohalm, and K. Ulbrich. Polyelectrolyte vectors for gene delivery: influence of cationic polymer on biophysical properties of complexes formed with DNA. Bioconjugate Chem. 10:993–1004 (1999).
C. Arigita, N. J. Zuidam, D. J. A. Crommelin, and W. E. Hennink. Association and dissociation characteristics of polymer/DNA complexes used for gene delivery. Pharm Res. 16:1534–1541 (1999).
J. K. W. Lam, Y. Ma, S. P. Armes, A. L. Lewis, T. Baldwin, and S. Stolnik. Phosphorylcholine-polycation diblock copolymers as synthetic vectors for gene delivery. J. Control. Release. 100:293–312 (2004).
M. H. Dufresne, and J. C. Leroux. Study of the micellization behavior of different order amino block copolymers with heparin. Pharm. Res. 21:160–169 (2004).
M. H. Dufresne, M. A. Gauthier, and J. C. Leroux. Thiol-functionalized polymeric micelles: From molecular recognition to improved mucoadhesion. Bioconjugate Chem. 16:1027–1033 (2005).
W. H. Heath, A. F. Senyurt, J. Layman, and T. E. Long. Charged polymers via controlled radical polymerization and their implications for gene delivery. Macromol. Chem. Phys. 208:1243–1249 (2007).
M. C. Deshpande, M. C. Garnett, M. Vamvakaki, L. Bailey, S. P. Armes, and S. Stolnik. Influence of polymer architecture on the structure of complexes formed by PEG-tertiary amine methacrylate copolymers and phosphorothioate oligonucleotide. J. Control. Release. 81:185–199 (2002).
M. Ranger, M. C. Jones, M. A. Yessine, and J. C. Leroux. From well-defined diblock copolymers prepared by a versatile atom transfer radical polymerization method to supramolecular assemblies. J. Polym. Sci., A, Polym. Chem. 39:3861–3874 (2001).
M. A. Yessine, C. Meier, H. U. Petereit, and J. C. Leroux. On the role of methacrylic acid copolymers in the intracellular delivery of antisense oligonucleotides. Eur. J. Pharm. Biopharm. 63:1–10 (2006).
M. A. Yessine, M. H. Dufresne, C. Meier, H. U. Petereit, and J. C. Leroux. Proton-actuated membrane-destabilizing polyion complex micelles. Bioconjugate Chem. 18:1010–1014 (2007).
S. Lenoir, C. Pagnoulle, C. Detrembleur, M. Galleni, and R. Jerome. New antibacterial cationic surfactants prepared by atom transfer radical polymerization. J. Polym. Sci., A, Polym. Chem. 44:1214–1224 (2006).
U. Rungsardthong, T. Ehtezazi, L. Bailey, S. P. Armes, M. C. Garnett, and S. Stolnik. Effect of polymer ionization on the interaction with DNA in nonviral gene delivery systems. Biomacromolecules. 4:683–690 (2003).
H. Dautzenberg, C. Konak, T. Reschel, A. Zintchenko, and K. Ulbrich. Cationic graft copolymers as carriers for delivery of antisense-oligonucleotides. Macromol. Biosci. 3:425–435 (2003).
M. Glodde, S. R. Sirsi, and G. J. Lutz. Physiochemical properties of low and high molecular weight poly(ethylene glycol)-grafted poly(ethylene imine) copolymers and their complexes with oligonucleotides. Biomacromolecules. 7:347–356 (2006).
A. Harada, H. Togawa, and K. Kataoka. Physicochemical properties and nuclease resistance of antisense-oligonucleotides entrapped in the core of polyion complex micelles composed of poly(ethylene glycol)–poly(l-lysine) block copolymers. Eur. J. Pharm. Sci. 13:35–42 (2001).
C. W. Scales, F. Q. Huang, N. Li, Y. A. Vasilieva, J. Ray, A. J. Convertine, and C. L. McCormick. Corona-stabilized interpolyelectrolyte complexes of siRNA with nonimmunogenic, hydrophilic/cationic block copolymers prepared by aqueous RAFT polymerization. Macromolecules. 39:6871–6881 (2006).
J. Jin, J. C. Achenbach, S. P. Zhu, and Y. F. Li. Complexation of well-controlled low-molecular weight polyelectrolytes with antisense oligonucleotides. Colloid Polym. Sci. 283:1197–1205 (2005).
M. Elsabahy, M. Zhang, S. M. Gan, K. C. Waldron, and J. C. Leroux. Synthesis and enzymatic stability of PEGylated oligonucleotide duplexes and their self-assemblies with polyamidoamine dendrimers. Soft Matter. 4:294–302 (2008).
G. Gaucher, M. H. Dufresne, V. P. Sant, N. Kang, D. Maysinger, and J. C. Leroux. Block copolymer micelles: preparation, characterization and application in drug delivery. J. Control. Release. 109:169–188 (2005).
O. Boussif, F. Lezoualc’h, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix, and J. P. Behr. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U. S. A. 92:7297–7301 (1995).
Acknowledgments
This work was financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Research Chair Program. M.H.D. further acknowledges graduate research scholarships from NSERC and Fonds Québécois de la Recherche sur la Nature et les Technologies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dufresne, MH., Elsabahy, M. & Leroux, JC. Characterization of Polyion Complex Micelles Designed to Address the Challenges of Oligonucleotide Delivery. Pharm Res 25, 2083–2093 (2008). https://doi.org/10.1007/s11095-008-9591-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9591-6