Abstract
Purpose
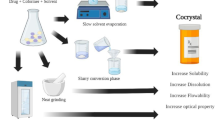
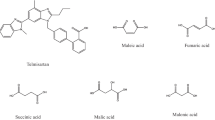
To develop a streamlined strategy for pharmaceutical cocrystal preparation without knowledge of the stoichiometric ratio by preparing and characterizing the cocrystals of myricetin (MYR) with four cocrystal coformers (CCF).
Methods
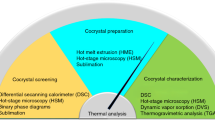
An approach based on the phase solubility diagram (PSD) was used for MYR cocrystals preparation and the solid-state properties were characterized by differential scanning calorimetry (DSC), fourier transform-infrared spectroscopy (FT-IR), powder X-ray diffraction (PXRD), and scanning electron microscopy (SEM). The ternary phase diagram (TPD) was constructed by combining the PSD and nuclear magnetic resonance (NMR) data. After that, the TPD was verified by traditional methods. The dissolution of MYR in the four cocrystals and pure MYR within three different media were also evaluated.
Results
A simple research method for MYR cocrystal preparation was obtained as follows: first, the PSD of MYR and CCF was constructed and analyzed; second, by transforming the curve in the PSD to a TPD, a region of pure cocrystals formation was exhibited, and then MYR cocrystals were prepared and identified by DSC, FT-IR, PXRD, and SEM; third, with the composition of the prepared cocrystal from NMR, the TPD of the MYR-CCF-Solvent system was constructed. The powder dissolution data showed that the solubility and dissolution rate of MYR was significantly enhanced by the cocrystals.
Conclusions
A novel strategy for pharmaceutical cocrystals preparation without knowledge of the stoichiometric ratio based on the TPD was established and MYR cocrystals were successfully prepared. The present study provides a systematic approach for pharmaceutical cocrystal generation, which benefits the development and application of cocrystal technology in drug delivery.












Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- CAF:
-
Caffeine
- CCF:
-
Cocrystal coformer
- CYA:
-
4-Cyanopyridine
- DSC:
-
Differential scanning calorimetry
- FT-IR:
-
Fourier transform-infrared spectroscopy
- HTS:
-
High throughput screening
- INM:
-
Isonicotinamide
- MYR:
-
Myricetin
- NIC:
-
Nicotinamide
- PSD:
-
Phase solubility diagram
- PXRD:
-
Powder X-ray diffraction
- SEM:
-
Scanning electron microscopy
- TPD:
-
Ternary phase diagram
- USP:
-
United states pharmacopeia
References
Eddleston MD, Sivachelvam S, Jones W. Screening for polymorphs of cocrystals: a case study. Cryst Eng Comm. 2013;15(1):175–81.
Qiao N, Li MZ, Schlindwein W, Malek N, Davies A, Trappitt G. Pharmaceutical cocrystals: an overview. Int J Pharm. 2011;419(1–2):1–11.
Seliger J, Zagar V. Nuclear quadrupole resonance characterization of carbamazepine cocrystals. Solid State Nucl Magn Reson. 2012;47–48(1):47–52.
Yamamoto K, Tsutsumi S, Ikeda Y. Establishment of cocrystal cocktail grinding method for rational screening of pharmaceutical cocrystals. Int J Pharm. 2012;437(1–2):162–71.
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Ong KC, Khoo HE. Biological effects of myricetin. Gen Pharmacol Vasc Sys. 1997;29(2):121–6.
Scheidt HA, Pampel A, Nissler L, Gebhardt R, Huster D. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectroscopy. Biochim Biophys Acta. 2004;1663(1–2):97–107.
Kim H, Choi J, Jung S. Inclusion complexes of modified cyclodextrins with some flavonols. J Incl Phenom Macrocycl Chem. 2009;64(1–2):43–7.
Mira L, Fernandez MT, Santos M, Rocha R, Florencio MH, Jennings KR. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002;36(11):1199–208.
Roedig-Penman A, Gordon MH. Antioxidant properties of myricetin and quercetin in oil and emulsions. J Am Oil Chem Soc. 1998;75(2):169–80.
Ma ZG, Liu TW. Myricetin facilitates potassium currents and inhibits neuronal activity of PVN neurons. Neurochem Res. 2012;37(7):1450–6.
Yao YS, Lin GB, Xie Y, Ma P, Li GW, Meng QC, et al. Preformulation studies of myricetin: a natural antioxidant flavonoid. Die Pharm. 2014;69(1):19–26.
Variankaval N, Wenslow R, Murry J, Hartman R, Helmy R, Kwong E, et al. Preparation and solid-state characterization of nonstoichiometric cocrystals off a phosphodiesterase-IV inhibitor annul L-tartaric acid. Cryst Growth Des. 2006;6(3):690–700.
Leung DH, Lohani S, Ball RG, Canfield N, Wang YL, Rhodes T, et al. Two novel pharmaceutical cocrystals of a development compound–screening, scale-up, and characterization. Cryst Growth Des. 2012;12(3):1254–62.
Arenas-Garcia JI, Herrera-Ruiz D, Mondragon-Vasquez K, Morales-Rojas H, Hopfl H. Modification of the supramolecular hydrogen-bonding patterns of acetazolamide in the presence of different cocrystal formers: 3:1, 2:1, 1:1, and 1:2 cocrystals from screening with the structural isomers of hydroxybenzoic acids, aminobenzoic acids, hydroxybenzamides, aminobenzamides, nicotinic acids, nicotinamides, and 2,3-dihydroxybenzoic acids. Cryst Growth Des. 2012;12(2):811–24.
Ando S, Kikuchi J, Fujimura Y, Ida Y, Higashi K, Moribe K, et al. Physicochemical characterization and structural evaluation of a specific 2:1 cocrystal of naproxen-nicotinamide. J Pharm Sci. 2012;101(9):3214–21.
Gangopadhyay P, Radhakrishnan TP. Visualizing supramolecular macrocyclic formations. Mol Cryst Liq Cryst. 2001;369(1):167–219.
Yenikaya C, Ogretir C. A quantum chemical study on structure of cocrystal of triphenylphosphine oxide and hydroquinone. J Mol Struct THEOCHEM. 2005;731(1–3):1–5.
Jayasankar A, Reddy LS, Bethune SJ, Rodriguez-Hornedo N. Role of cocrystal and solution chemistry on the formation and stability of cocrystals with different stoichiometry. Cryst Growth Des. 2009;9(2):889–97.
Chiarella RA, Davey RJ, Peterson ML. Making co-crystals–the utility of ternary phase diagrams. Cryst Growth Des. 2007;7(7):1223–6.
Rodriguez-Hornedo N, Nehru SJ, Seefeldt KF, Pagan-Torres Y, Falkiewicz C. Reaction crystallization of pharmaceutical molecular complexes. Mol Pharm. 2006;3(3):362–7.
Good DJ, Rodriguez-Hornedo N. Cocrystal eutectic constants and prediction of solubility behavior. Cryst Growth Des. 2010;10(3):1028–32.
Nehm SJ, Rodriguez-Spong B, Rodriguez-Hornedo N. Phase solubility diagrams of cocrystals are explained by solubility product and solution complexation. Cryst Growth Des. 2006;6(2):592–600.
Zhang S, Rasmuson AC. Thermodynamics and crystallization of the theophylline-glutaric acid cocrystal. Cryst Growth Des. 2013;13(3):1153–61.
Grossjohann C, Eccles KS, Maguire AR, Lawrence SE, Tajber L, Corrigan OI, et al. Characterisation, solubility and intrinsic dissolution behaviour of benzamide: dibenzyl sulfoxide cocrystal. Int J Pharm. 2012;422(1–2):24–32.
Childs SL, Rodriguez-Hornedo N, Reddy LS, Jayasankar A, Maheshwari C, McCausland L, et al. Screening strategies based on solubility and solution composition generate pharmaceutically acceptable cocrystals of carbamazepine. Cryst Eng Comm. 2008;10(7):856–64.
Ainouz A, Authelin JR, Billot P, Lieberman H. Modeling and prediction of cocrystal phase diagrams. Int J Pharm. 2009;374(1–2):82–9.
Croker DM, Foreman ME, Hogan BN, Maguire NM, Elcoate CJ, Hodnett BK, et al. Understanding the p-toluenesulfonamide/triphenylphosphine oxide crystal chemistry: a new 1:1 cocrystal and ternary phase diagram. Cryst Growth Des. 2012;12(2):869–75.
Seaton CC, Parkin A, Wilson CC, Blagden N. Controlling the formation of benzoic acid: isonicotinamide molecular complexes. Cryst Growth Des. 2009;9(1):47–56.
Eddleston MD, Lloyd GO, Jones W. Cocrystal dissociation and molecular demixing in the solid state. Chem Commun. 2012;48(65):8075–7.
Vangala VR, Chow PS, Tan RBH. Co-crystals and co-crystal hydrates of the antibiotic nitrofurantoin: structural studies and physicochemical properties. Cryst Growth Des. 2012;12(12):5925–38.
Schultheiss N, Newman A. Pharmaceutical cocrystals and their physicochemical properties. Cryst Growth Des. 2009;9(6):2950–67.
Stanton MK, Bak A. Physicochemical properties of pharmaceutical co-crystals: a case study of ten AMG 517 co-crystals. Cryst Growth Des. 2008;8(10):3856–62.
Weyna DR, Shattock T, Vishweshwar P, Zaworotko MJ. Synthesis and structural characterization of cocrystals and pharmaceutical cocrystals: mechanochemistry vs. slow evaporation from solution. Cryst Growth Des. 2009;9(2):1106–23.
Espinosa-Lara JC, Guzman-Villanueva D, Arenas-Garcia JI, Herrera-Ruiz D, Rivera-Islas J, Roman-Bravo P, et al. Cocrystals of active pharmaceutical ingredients-praziquantel in combination with oxalic, malonic, succinic, maleic, fumaric, glutaric, adipic, and pimelic acids. Cryst Growth Des. 2013;13(1):169–85.
Mohamed S, Tocher DA, Price SL. Computational prediction of salt and cocrystal structures-does a proton position matter? Int J Pharm. 2011;418(2):187–98.
Alhalaweh A, George S, Basavoju S, Childs SL, Rizvi SAA, Velaga SP. Pharmaceutical cocrystals of nitrofurantoin: screening, characterization and crystal structure analysis. Cryst Eng Comm. 2012;14(15):5078–88.
Guo K, Sadiq G, Seaton C, Davey R, Yin QX. Co-crystallization in the caffeine/maleic acid system: lessons from phase equilibria. Cryst Growth Des. 2010;10(1):268–73.
Yamashita H, Hirakura Y, Yuda M, Teramura T, Terada K. Detection of cocrystal formation based on binary phase. Pharm Res. 2013;30(1):70–80.
Thakuria R, Delori A, Jones W, Lipert MP, Roy L, Rodriguez-Hornedo N. Pharmaceutical cocrystals and poorly soluble drugs. Int J Pharm. 2013;453(1):101–25.
Bethune SJ, Huang N, Jayasankar A, Rodriguez-Hornedo N. Understanding and predicting the effect of cocrystal components and pH on cocrystal solubility. Cryst Growth Des. 2009;9(9):3976–88.
Alhalaweh A, Roy L, Rodriguez-Hornedo N, Velaga SP. pH-dependent solubility of indomethacin-saccharin and carbamazepine-saccharin cocrystals in aqueous media. Mol Pharm. 2012;9(9):2605–12.
Reddy LS, Bethune SJ, Kampf JW, Rodriguez-Hornedo N. Cocrystals and salts of gabapentin: pH dependent cocrystal stability and solubility. Cryst Growth Des. 2009;9(1):378–85.
Gao YA, Zu H, Zhang JJ. Enhanced dissolution and stability of adefovir dipivoxil by cocrystal formation. J Pharm Pharmacol. 2011;63(4):483–90.
Rahman Z, Samy R, Sayeed VA, Khan MA. Physicochemical and mechanical properties of carbamazepine cocrystals with saccharin. Pharm Dev Technol. 2012;17(4):457–65.
Bruni G, Maietta M, Maggi L, Mustarelli P, Ferrara C, Berbenni V, et al. Preparation and physicochemical characterization of acyclovir cocrystals with improved dissolution properties. J Pharm Sci. 2013;102(11):4079–86.
Bolla G, Sanphui P, Nangia A. Solubility advantage of tenoxicam phenolic cocrystals compared to salts. Cryst Growth Des. 2013;13(5):1988–2003.
Maddileti D, Jayabun SK, Nangia A. Soluble cocrystals of the xanthine oxidase inhibitor febuxostat. Cryst Growth Des. 2013;13(7):3188–96.
Luo YH, Sun BW. Pharmaceutical co-crystals of pyrazinecarboxamide (PZA) with various carboxylic acids: crystallography, hirshfeld surfaces, and dissolution study. Cryst Growth Des. 2013;13(5):2098–106.
Shiraki K, Takata N, Takano R, Hayashi Y, Terada K. Dissolution improvement and the mechanism of the improvement from cocrystallization of poorly water-soluble compounds. Pharm Res. 2008;25(11):2581–92.
Childs SL, Kandi P, Lingireddy SR. Formulation of a danazol cocrystal with controlled supersaturation plays an essential role in improving bioavailability. Mol Pharm. 2013;10(8):3112–27.
Acknowledgments and Disclosures
This study was sponsored by the National Science Foundation of China (81303304), the Innovation Program of the Shanghai Municipal Education Commission (14YZ057), the Specialized Research Fund for the Doctoral Program of Higher Education (20133107120006), and the Nano-specific Project of the Shanghai Science and Technology Commission (12 nm0502400).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
SFig. 1
FTIR results of pure MYR. (GIF 323 kb)
SFig. 2
FTIR results of pure CAF. (GIF 349 kb)
SFig. 3
FTIR results of MYR-CAF cocrystal. (GIF 321 kb)
SFig. 4
FTIR results of pure NIC. (GIF 349 kb)
SFig. 5
FTIR results of MYR-NIC cocrystal. (GIF 122 kb)
SFig. 6
FTIR results of pure INM. (GIF 329 kb)
SFig. 7
FTIR results of MYR-INM cocrystal. (GIF 318 kb)
SFig. 8
FTIR results of pure CYA. (GIF 384 kb)
SFig. 9
FTIR results of MYR-CYA cocrystal. (GIF 378 kb)
SFig. 10
PXRD patterns of pure MYR (a), MYR-CAF cocrystal (b), pure CAF (c). (GIF 532 kb)
SFig. 11
PXRD patterns of pure MYR (a), MYR-NIC cocrystal (d), pure NIC (e). (GIF 529 kb)
SFig. 12
PXRD patterns of pure MYR (a), MYR-INM cocrystal (f), pure INM (g). (GIF 542 kb)
SFig. 13
PXRD patterns of pure MYR (a), MYR-CYA cocrystal (h), pure CYA (i). (GIF 500 kb)
Rights and permissions
About this article
Cite this article
Hong, C., Xie, Y., Yao, Y. et al. A Novel Strategy for Pharmaceutical Cocrystal Generation Without Knowledge of Stoichiometric Ratio: Myricetin Cocrystals and a Ternary Phase Diagram. Pharm Res 32, 47–60 (2015). https://doi.org/10.1007/s11095-014-1443-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1443-y