Abstract
Purpose
It is hypothesized that sodium acetate (SA) can be used for in situ coating of drug loaded chitosan NPs for improved physico-chemical properties.
Methods
Tenofovir (TFV) is used as a model drug. Uncoated chitosan NPs are prepared by ionic gelation. SA is generated in situ from half neutralization of acetic acid with sodium hydroxide, and coats chitosan NPs during freeze-drying. The NPs' physico-chemical properties [e.g., particle mean diameters (PMD) zeta potential (ζ), EE%, drug release profile, morphology] are characterized by dynamic light scattering, spectrophotometry, Korsmeyer-Peppas model, transmission electron microscopy (TEM), respectively. Melting point (MP), non-aqueous titration, Fourier transform infrared (FTIR) analysis, and powder X-ray diffractometry (XRD) pattern evaluate the SA coated chitosan NPs. The NPs' cytotoxicity on macrophages Raw 264.7 is assessed by neutral red, resazurin, nitrite oxide (NO) and cytokines assays.
Results
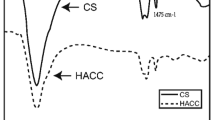
Collectively, FTIR, ζ, XRD, MP, and TEM data confirm that SA coats chitosan NPs. The PMD range is 136–348 nm (uncoated) and 171–379 nm (coated NPs). The ζ values range is +24.3–28.5 mV (uncoated) and 0.1–3.1 mV (coated NPs). The EE% ranges from 5.5 to 11.7% (uncoated NPs) and increased up to 86.3-92.7%(8-17-fold) after coating. The SA also prevents NPs aggregation during the freeze-drying and aqueous dispersion. The core-shell NPs exhibited a sustain release of TFV following anomalous transport mechanism (R2 ~ 0.99). The coated NPs are non-cytotoxic (cell viability ~100%) and without any proinflammatory response.
Conclusions
This SA coating chitosan NPs mechanism may be useful for (i) efficient encapsulation, (ii) stabilizing colloidal dispersions, (iii) controlling the release and solubility of bioactive agents.










Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- BCS:
-
Biopharmaceutics classification system
- DPBS:
-
Dulbecco’s phosphate buffered saline
- DMEM:
-
Dulbecco’s modified eagle medium
- EE %:
-
Percent encapsulation efficiency
- F:
-
Formulation
- FBS:
-
Fetal bovine serum
- FTIR:
-
Fourier transform infrared
- HSD:
-
Honestly significant difference
- HVF:
-
Human vaginal fluid
- IL:
-
Interleukin
- LM:
-
Lithium methoxide
- LPS:
-
Lipopolysaccharide
- M:
-
Molar mass
- Mc:
-
Molar mass corrected
- NO:
-
Nitric oxide
- NPs:
-
Nanoparticles
- NR:
-
Neutral red
- PA:
-
Perchloric acid
- PDI:
-
Polydispersity index
- PMD:
-
Particle mean diameters
- SA:
-
Sodium acetate
- SAA:
-
Sodium acetate anhydrous
- SAT:
-
Sodium acetate trihydrate
- SD:
-
Sodium diacetate
- TEM:
-
Transmission electron microscopy
- TFV:
-
Tenofovir
- TPP:
-
Polyanion triphosphate
- V:
-
Volume
- XRD:
-
Powder X-ray diffractometry
- ζ:
-
Zeta potential
References
Lee YL, Cesario T, Owens J, Shanbrom E, Thrupp LD. Antibacterial activity of citrate and acetate. Nutrition. 2002;18(7–8):665–6.
Frech G, Allen Jr LV, Stiles ML, Levinson RS. Sodium acetate as a preservative in protein hydrolysate solutions. Am J Hosp Pharm. 1979;36(12):1672–5.
Karaca H, Perez-Gago MB, Taberner V, Palou L. Evaluating food additives as antifungal agents against Monilinia fructicola in vitro and in hydroxypropyl methylcellulose-lipid composite edible coatings for plums. Int J Food Microbiol. 2014;179:72–9.
Costa C, Conte A, Del Nobile MA. Effective preservation techniques to prolong the shelf life of ready-to-eat oysters. J Sci Food Agric. 2014;94(13):2661–7.
Millard S, Larson H, Henry JF. Process of producing sodium or potassium acetate, propionate or butyrate. 1959; US Patent 2,895,990.
Zheng H, Tang C, Yin C. Exploring advantages/disadvantages and improvements in overcoming gene delivery barriers of amino Acid modified trimethylated chitosan. Pharm Res. 2015;32(6):2038–50.
Meng J, Sturgis TF, Youan BB. Engineering tenofovir loaded chitosan nanoparticles to maximize microbicide mucoadhesion. Eur J Pharm Sci. 2011;44(1–2):57–67.
Ngo AN, Ezoulin MJ, Youm I, Youan BB. Optimal Concentration of 2,2,2-Trichloroacetic Acid for Protein Precipitation Based on Response Surface Methodology. J Anal Bioanal Tech. 2014; 5(4).
Date AA, Destache CJ. A review of nanotechnological approaches for the prophylaxis of HIV/AIDS. Biomaterials. 2013;34(26):6202–28.
Rautio J, Mannhold R, Kubinyl H, Folkers G. Prodrugs and Targeted Delivery. Wiley VCH: Hoboken; 2010.
Solinova V, Kasicka V, Sazelova P, Holy A. Chiral analysis of anti-acquired immunodeficiency syndrome drug, 9-(R)-[2-(phosphonomethoxy)propyl]adenine (tenofovir), and related antiviral acyclic nucleoside phosphonates by CE using beta-CD as chiral selector. Electrophoresis. 2009;30(12):2245–54.
Wang X, Zheng C, Wu Z, Teng D, Zhang X, Wang Z, Li C. Chitosan-NAC nanoparticles as a vehicle for nasal absorption enhancement of insulin. J Biomed Mater Res B Appl Biomater Part B, Appl Biomat. 2009;88(1):150–61.
Rampino A, Borgogna M, Blasi P, Bellich B, Cesaro A. Chitosan nanoparticles: preparation, size evolution and stability. Int J Pharm. 2013;455(1–2):219–28.
Cullity BD, Stock SR. Elements X-ray Diffraction. Prentice-hall, Inc: Upper Saddle River; 2001.
Fritz JS. Titration of Bases in Nonaqueous Solvents. Anal Chem. 1950; 22(8).
Bruss DB, Harlow GA. Titration of Weak Acids in Nonaqueous Solvents: Potentiometric Studies in Inert Solvents. Anal Chem. 1958; 30(11).
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33.
Wadajkar AS, Kadapure T, Zhang Y, Cui W, Nguyen KT, Yang J. Dual-imaging enabled cancer-targeting nanoparticles. Adv Health Mater. 2012;1(4):450–6.
Panda SK, Kumar S, Tupperwar NC, Vaidya T, George A, Rath S, Bal V, Ravindran B. Chitohexaose activates macrophages by alternate pathway through TLR4 and blocks endotoxemia. PLoS Pathog. 2012;8(5):e1002717.
Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166(6):3873–81.
Vega-Avila E, Pugsley MK. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc West Pharmacol Soc. 2011;54:10–4.
Hannah RW, Mayo D, Miller FA. Course notes on the interpretaion of Infrared and Raman Spectra. Willey & Sons Publication 2004;:210–213.
Cho J, Heuzey MC, Begin A, Carreau PJ. Physical gelation of chitosan in the presence of beta-glycerophosphate: the effect of temperature. Biomacromolecules. 2005;6(6):3267–75.
van Beilen JW. Teixeira de Mattos MJ, Hellingwerf KJ, Brul S. Distinct effects of sorbic acid and acetic acid on the electrophysiology and metabolism of Bacillus subtilis. Appl Environ Microbiol. 2014;80(19):5918–26.
Po HN, Senozan NM. The Henderson–Hasselbalch equation: its history and limitations. J Chemical Educ. 2001;78(11):1499–503.
Bigucci F, Abruzzo A, Vitali B, Saladini B, Cerchiara T, Gallucci MC, Luppi B. Vaginal inserts based on chitosan and carboxymethylcellulose complexes for local delivery of chlorhexidine: preparation, characterization and antimicrobial activity. Int J Pharm. 2015;478(2):456–63.
Mi F, Shyu S, Wong T, Jang S, Lee S, Lu K. Chitosan–polyelectrolyte complexation for the preparation of gel beads and controlled release of anticancer drug. II. effect of pH-dependent ionic crosslinking or interpolymer complex using tripolyphosphate or polyphosphate as reagent. J Appl Pol Sci. 1999;74:1093–107.
Holleman AF, Wiberg E. Inorganic chemistry. Orlando: Harcourt Science and Technology company; 2001.
Magder S, Emami A. Practical approach to physical-chemical acid–base management. Stewart at the bedside. Ann Am Thorac Soc. 2015;12(1):111–7.
Hu W, Ding L, Cao J, Liu L, Wei Y, Fang Y.. Protein binding-induced surfactant aggregation variation: a new strategy of developing fluorescent aqueous sensor for proteins. ACS Appl Mater Interf. 2015;7(8):4728–36.
Padhiyar TC, Thakore SB. Recovery of acetic acid from effluent via freeze crystallization. Int J Sci Eng Technol. 2013;2(4):211–5.
Cengel YAT, Robert H. fundamentals of thermal-fluid sciences. Boston: McGraw-Hill; 2004.
Agreda VH, Zoeller JR. Acetic acid and its derivatives. New York: Marcel Dekker; 2001.
Atkins P, de Paula J. Atkin's physical chemistry. 9th ed. New York: W.H Freeman and company; 2010.
Minofar B, Jungwirth P , Das MR, Kunz W, Mahiuddin S. Propensity of formate, acetate, benzoate, and phenolate for the aqueous solution/vapor interface: surface tension measurements and molecular dynamics simulations. J Phys Chem. 2007;111:8242–7.
Barrow MJ, Murdoch Currie K, Muir W, Clare Speakman J, White DNJ. (Crystal structures of some acid salts of monobasic acids. part XVII. Structure of sodium hydrogen diacetate, redetermined by neutron diffraction. J Chem Soc. Perkin 2:15–17.
Cameron TS, Mannan KM, Rahaman MO. The crystal structure of sodium acetate trihydrate. Acta Crystal B. 1976;32:87–90.
Cullity BD, Stock SR (2001) Elements x-ray diffraction. 2001; Book, ISBN 0-201-61091-4 3 296.
Zambito Y, Pedreschi E, Di-Colo G. Is dialysis a reliable method for studying drug release from nanoparticulate systems?-A case study. Int J Pharm. 2012;434(1–2):28–34.
Chavanpatil MD, Jain P, Chaudhari S, Shear R, Vavia PR. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int J Pharm. 2006;316(1–2):86–92.
Smith A, Perelman M, Hinchcliffe M. Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum Vac Immunother. 2014;10(3):797–807.
Lopez-Garcia J, Lehocky M, Humpolicek P, Saha P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: extent of cytotoxicity, cell viability and proliferation. J Funct Biomater. 2014;5(2):43–57.
Introini A, Vanpouille C, Lisco A, Grivel JC, Margolis L. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathog. 2013;9(2):e1003148.
ACKNOWLEDGMENTS AND DISCLOSURES
This work is supported by award number R01 AI087304, from the National Institute of Allergic and Infectious Diseases (Bethesda, MD, USA). The content is solely the responsibility of the authors and does not necessarily represent the official view of the national Institute of Allergy and infectious Diseases or the national Institutes of Health. The work is patent pending, provisional patent application #62/173,772.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Colorless aqueous soltuion of chitosan dissolved in 2% v/v glacial acetic acid (A), and formation of uncoated chitosan NPs through ionic gelation technique. The color of the solution changes from colorless to milky (Tyndall effect) (B). (GIF 305 kb)
Fig. S2
Aggregation of chitosan NPs for “blank “, F1, F2, F3 formulation using the classical ionic gelation process after freeze drying respectively without use of cryoprotectant (A). It is noteworhty that SA coated chitosan NPs prevents their aggregation during freeze-drying process without also use of cryprotectant (B). (GIF 302 kb)
Fig. S3
Uncoated chitosan NPs forming a ‘cake’ using the classical ionic gelation process cannot be dispersed in deionized water for the blank and the three formulations (F1, F2 and F3) formulation, respectively after freeze drying without the use of cryprotectant and surfactant (A). Sodium acetate (SA) coated chitosan NPs can be easily dispersed (~10 mg/mL) in deionized water due to the hydrotropic properties of SA without also the use of cryoprotant and surfactant (B). (GIF 264 kb)
Rights and permissions
About this article
Cite this article
Ngo, A.N., Ezoulin, M.J.M., Murowchick, J.B. et al. Sodium Acetate Coated Tenofovir-Loaded Chitosan Nanoparticles for Improved Physico-Chemical Properties. Pharm Res 33, 367–383 (2016). https://doi.org/10.1007/s11095-015-1795-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1795-y