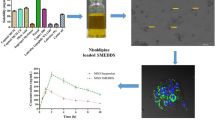
Abstract
Purpose
The aim of the study was to evaluate organogel nanoparticles as a lipophilic vehicle to increase the oral bioavailability of poorly soluble compounds. Efavirenz (EFV), a Biopharmaceutical Classification System (BCS) Class II, was used as drug model.
Methods
Organogel nanoparticles loaded with EFV were formulated with sunflower oil, 12-hydroxystearic acid (HSA) and polyvinyl alcohol (PVA). Various parameters have been investigated in the current study such as (i) the release profile of organogel assessed by USP 4 cell flow dialysis, (ii) the impact of organogel on intestinal absorption, using Caco-2 cells as in vitro model and jejunum segments as ex vivo assay and (iii) the bioavailability of organogel following oral pharmacokinetic study.
Results
250–300 nm spherical particles with a final concentration of 4.75 mg/mL drug loading were obtained, corresponding to a thousand fold increase in EFV solubility, combined to a very high encapsulation efficiency (>99.8%). Due to rapid diffusion, drug was immediately released from the nanoparticles. The biopharmaceutical evaluation on ex vivo jejunum segments demonstrated an increased absorption of EFV from organogel nanoparticles compare to a native EFV suspension. In vitro assays combining Caco-2 cell cultures with TEM and confocal microscopy demonstrated passive diffusion, while paracellular integrity and endocytosis activity remain expelled. Oral pharmacokinetics of EFV organogel nanoparticles improve oral bioavailability (Fr: 249%) and quick absorption compared to EFV suspension.
Conclusion
Organogel nanoparticles increase the bioavailability of BCS Class II drugs. The main phenomena is simply oil transfer from the gelled particles through the cell membrane.












Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- EFV:
-
Efavirenz
- NR:
-
Nile Red
- PVA:
-
Polyvinyl alcohol
- HAS:
-
Hydroxystearic acid
- DLS:
-
Dynamic Light Scattering
- TEM:
-
Transmission electron microscopy
- DSC:
-
Differential scanning calorimetry
- EE:
-
Encapsulation efficiency
- SGF:
-
Simulated gastric fluid
- SIF:
-
Simulated intestinal fluid
- Tmax:
-
Time to maximum concentration
- Cmax:
-
Maximum plasma concentration
- SD:
-
Standard deviation
References
Oprea TI. Current trends in lead discovery: are we looking for the appropriate properties? Mol Divers. 2000;5:199–208.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25.
Di L, Fish PV, Mano T. Bridging solubility between drug discovery and development. Drug Discov Today. 2012;17:486–95. https://doi.org/10.1016/j.drudis.2011.11.007.
Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13:519–47. https://doi.org/10.1208/s12248-011-9290-9.
Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, et al. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013;65:315–499. https://doi.org/10.1124/pr.112.005660.
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–53. https://doi.org/10.1016/j.apsb.2015.07.003.
Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–48. https://doi.org/10.1038/nrd2197.
Mu H, Holm R, Müllertz A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int J Pharm. 2013;453:215–24. https://doi.org/10.1016/j.ijpharm.2013.03.054.
Kesisoglou F, Panmai S, Wu Y. Nanosizing — Oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59:631–44. https://doi.org/10.1016/j.addr.2007.05.003.
Merisko-Liversidge EM, Liversidge GG. Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol Pathol. 2008;36:43–8. https://doi.org/10.1177/0192623307310946.
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–84.
McClements DJ, Rao J. Food-grade Nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr. 2011;51:285–330. https://doi.org/10.1080/10408398.2011.559558.
Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interf Sci. 2004;108–109:303–18.
Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–55.
Freitas C, Müller RH. Correlation between long-term stability of solid lipid nanoparticles (SLN™) and crystallinity of the lipid phase. Eur J Pharm Biopharm. 1999;47:125–32.
Das S, Ng WK, Tan RBH. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci. 2012;47:139–51.
Kirilov P, Lukyanova L, Franceschi-Messant S, Perier V, Perez E, Rico-Lattes I. A new type of colloidal dispersions based on nanoparticles of gelled oil. Colloids Surf Physicochem Eng Asp. 2008;328:1–7.
Martin B, Brouillet F, Franceschi S, Perez E. Evaluation of Organogel nanoparticles as drug delivery system for lipophilic compounds. AAPS PharmSciTech. 2017;18:1261–9. https://doi.org/10.1208/s12249-016-0587-y.
European Pharmacopoeia, 8th ed., European Directorate for the Quality of Medicines & Healthcare, Council of Europe, Strasbourg, France, 2012.
Garrait G, Jarrige JF, Blanquet S, Beyssac E, Cardot JM, Alric M. Gastrointestinal absorption and urinary excretion of trans -Cinnamic and p -Coumaric acids in rats. J Agric Food Chem. 2006;54:2944–50. https://doi.org/10.1021/jf053169a.
Pinto EC, Cabral LM, de Sousa VP. Development of a Discriminative Intrinsic Dissolution Method for Efavirenz. Dissolution Technol. 2014;21:31–40. https://doi.org/10.14227/DT210214P31.
Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the Weibull function for the discernment of drug release mechanisms. Int J Pharm. 2006;309:44–50. https://doi.org/10.1016/j.ijpharm.2005.10.044.
Küchler S, Abdel-Mottaleb M, Lamprecht A, Radowski MR, Haag R, Schäfer-Korting M. Influence of nanocarrier type and size on skin delivery of hydrophilic agents. Int J Pharm. 2009;377:169–72. https://doi.org/10.1016/j.ijpharm.2009.04.046.
Sun H, Chow EC, Liu S, Du Y, Pang KS. The Caco-2 cell monolayer: usefulness and limitations. Expert Opin Drug Metab Toxicol. 2008;4:395–411. https://doi.org/10.1517/17425255.4.4.395.
Mahmoudi M, Simchi A, Imani M. Cytotoxicity of uncoated and polyvinyl alcohol coated superparamagnetic iron oxide nanoparticles. J Phys Chem C. 2009;113:9573–80.
Petersen S, Steiniger F, Fischer D, Fahr A, Bunjes H. The physical state of lipid nanoparticles influences their effect on in vitro cell viability. Eur J Pharm Biopharm. 2011;79:150–61. https://doi.org/10.1016/j.ejpb.2011.03.022.
Boudier A, Kirilov P, Franceschi-Messant S, Haouaria B, Hadioui L, Roques R, et al. Evaluation of biocompatible stabilised gelled soya bean oil nanoparticles as new hydrophobic reservoirs. J Microencapsul. 2010;27:682–92.
Chiappetta DA, Hocht C, Taira C, Sosnik A. Oral pharmacokinetics of the anti-HIV efavirenz encapsulated within polymeric micelles. Biomaterials. 2011;32:2379–87. https://doi.org/10.1016/j.biomaterials.2010.11.082.
Wagner JG, Nelson E. Per cent absorbed time plots derived from blood level and/or urinary excretion data. J Pharm Sci. 1963;52:610–1.
Bahal SM, Romansky JM, Alvarez FJ. Medium chain triglycerides as vehicle for palatable Oral liquids. Pharm Dev Technol. 2003;8:111–5.
Patel GV, Patel VB, Pathak A, Rajput SJ. Nanosuspension of efavirenz for improved oral bioavailability: formulation optimization, in vitro, in situ and in vivo evaluation. Drug Dev Ind Pharm. 2014;40:80–91. https://doi.org/10.3109/03639045.2012.746362.
Jain S, Sharma JM, Agrawal AK, Mahajan RR. Surface stabilized Efavirenz nanoparticles for Oral bioavailability enhancement. J Biomed Nanotechnol. 2013;9:1862–74. https://doi.org/10.1166/jbn.2013.1683.
Avachat AM, Parpani SS. Formulation and development of bicontinuous nanostructured liquid crystalline particles of efavirenz. Colloids Surf B Biointerfaces. 2015;126:87–97. https://doi.org/10.1016/j.colsurfb.2014.12.014.
Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495:439–46. https://doi.org/10.1016/j.ijpharm.2015.09.014.
Chiappetta DA, Hocht C, Taira C, Sosnik A. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailability. Nanomed. 2010;5:11–23. https://doi.org/10.2217/nnm.09.90.
Kou L, Sun J, Zhai Y, He Z. The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J Pharm Sci. 2013;8:1–10. https://doi.org/10.1016/j.ajps.2013.07.001.
S. Snipstad, S. Westrøm, Y. Mørch, M. Afadzi, A. K. A. Aslund C . De Lange Davies, Contact-mediated intracellular delivery of hydrophobic drugs from polymeric nanoparticles, Cancer Nanotechnol. 5 (2014) 1.
Klymchenko AS, Roger E, Anton N, Anton H, Shulov I, Vermot J, et al. Highly lipophilic fluorescent dyes in nano-emulsions: towards bright non-leaking nano-droplets. RSC Adv. 2012;2:11876–86. https://doi.org/10.1039/c2ra21544f.
Hofmann D, Messerschmidt C, Bannwarth MB, Landfester K, Mailänder V. Drug delivery without nanoparticle uptake: delivery by a kiss-and-run mechanism on the cell membrane. Chem Commun. 2014;50:1369–71. https://doi.org/10.1039/C3CC48130A.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martin, B., Garrait, G., Beyssac, E. et al. Organogel Nanoparticles as a New Way to Improve Oral Bioavailability of Poorly Soluble Compounds. Pharm Res 37, 92 (2020). https://doi.org/10.1007/s11095-020-02808-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02808-w