Abstract
Purpose
Rice is a staple crop worldwide and a silicon (Si) hyperaccumulator with Si levels reaching 5–10% of its mass; this can result in desilication and Si-deficiency if plant residues are not managed correctly. Rice is also uniquely subject to arsenic (As) and cadmium (Cd) contamination depending on soil conditions. Our goal is to quantify the effects of rice husk (a Si-rich milling byproduct) amendments and different water management strategies on rice uptake of Si, As, and Cd.
Methods
We employed 4 husk amendment treatments: Control (no husk), Husk (untreated husk), Biochar (husk pyrolyzed at 450 °C), and CharSil (husk combusted at > 1000 °C). Each of these amendments was studied under nonflooded, alternate wetting and drying (AWD), and flooded water management in a pot study. Porewater chemistry and mature plant elemental composition were measured.
Results
Husk and Biochar treatments, along with flooding, increased porewater and plant Si. Vegetative tissue As decreased with increasing porewater Si, but grain As and plant Cd were primarily controlled by water management. Grain As and Cd were inversely correlated and are simultaneously minimized in a redox potential (Eh) range of 225–275 mV in the studied soil. Ferrihydrite in root iron plaque decreased As translocation from porewater to grain, but amendments were not able to increase plaque ferrihydrite content.
Conclusion
We conclude moderate husk amendment rates (i.e., 4 years’ worth) with minimal pretreatment strongly increases rice Si content but may not be sufficient to decrease grain As in low Si and As soil.
Similar content being viewed by others
Introduction
Rice is the dominant source of calories for over half the world population (Seck et al. 2012), and demand is increasing each year, especially in developing countries (Chauhan et al. 2017). Rice is also a unique crop in that it accumulates high amounts (5–10%) of silicon (Si) in its tissues (Jones and Handreck 1967; Epstein 1994). Silicon is considered a beneficial nutrient for rice because it increases disease, pest, and stress resistance (Epstein 1994; N.K. Savant 1997; Voogt and Sonneveld 2001; Ma and Takahashi 2002; Ma 2004). Silicon can also increase grain yield (N.K. Savant 1997; Savant et al. 1997; Korndorfer and Lepsch 2001; Li et al. 2009b; Klotzbücher et al. 2015), particularly under arsenic stress (Teasley et al. 2017; Limmer et al. 2018b). Although Si is a ubiquitous component of soils, most silicate minerals are sparingly soluble. Managing plant-availability of Si in rice paddies is therefore crucial to ensure high crop productivity.
The Si present within plant tissues is typically found as amorphous SiO2 (Ma and Yamaji 2006), which has ~ 10 × higher solubility than crystalline SiO2 minerals such as quartz (Gunnarsson and Arnórsson 2000; Fraysse et al. 2006, 2009). This plant-opaline SiO2 pool plays an outsized role in the Si cycle, especially in surface soils (Alexandre et al. 1997; Struyf et al. 2010; Seyfferth et al. 2013; Cornelis and Delvaux 2016). Rice husk is an inedible covering on rice grains which is especially rich in plant-opaline Si (Li et al. 2013) and thus can serve as a source of Si for plant growth (Seyfferth et al. 2013, 2016, 2019a; Teasley et al. 2017; Limmer et al. 2018a; Linam et al. 2021). Husk is currently an underutilized byproduct which accumulates at rice mills (Minami and Saka 2005) and is sometimes burned to ash or pyrolyzed/charred (combusted in oxygen-limited conditions) to biochar at a wide range of temperatures to reduce its mass (Koyama et al. 2015). In general, dissolution kinetics of SiO2 from rice husk slow with increasing pyrolysis temperature (Linam et al. 2021), and solubility is decreased by burning to ash (Seyfferth et al. 2016; Teasley et al. 2017; Limmer et al. 2018a). Removing, pyrolyzing, or burning of rice husk, along with straw removal and desilication due to weathering, can cause significant loss of available Si from paddy soils (Berthelsen et al. 2003; Haynes 2014; Klotzbücher et al. 2015; Hughes et al. 2020). Reincorporation of rice straw has negative impacts such as increased methane production, but reincorporation of rice husk acts as a slow-release fertilizer and continues to provide Si for several years after incorporation (Penido et al. 2016; Teasley et al. 2017; Wu et al. 2020; Linam et al. 2021). It remains unknown how soil incorporation of unaltered rice husk affects plant growth and yield compared to incorporation of husk charred at different temperatures.
In addition to benefiting rice in terms of yield and stress resistance, Si is also closely associated with arsenic (As) uptake. The most dominant form of As in paddy soil solution (arsenite, As(III)) enters rice roots through the highly efficient Si transporters Lsi1 and Lsi2 (Jian et al. 2006; Ma et al. 2007, 2008), and the second most dominant form (dimethylarsinic acid (DMA)) also shares Lsi2 (Li et al. 2009a). Adequate supply of Si can decrease transporter number and activity, which decreases As uptake and toxicity—this has been shown in hydroponic and paddy rice studies (Guo et al. 2005; Bogdan and Schenk 2008; Ma et al. 2008; Zhao et al. 2009; Seyfferth and Fendorf 2012; Seyfferth et al. 2016, 2018, 2019a; Limmer et al. 2018b, a). Any practice altering Si availability to rice likely also affects As uptake, and thus increasing Si nutrition has been promoted as a sustainable way to lower As uptake by rice (Runkle et al. 2021).
Another way to affect the plant-uptake of toxic metal(loid)s in rice is through water management. The flooded conditions typically used in rice production result in low soil redox potential (Eh) and reductive dissolution of As-bearing iron (Fe) oxide minerals. This allows adsorbed arsenate (As(V)) to be reduced to the much more soluble As(III), which is more plant-available; As(III) can subsequently be methylated to the less (human-) toxic but more translocatable (Zhao et al. 2009, 2013; Carey et al. 2010, 2011; Zheng et al. 2013; Seyfferth et al. 2018) forms monomethylarsonic acid (MMA) or DMA. One method of lessening As uptake is allowing soil to dry—either periodically as in alternate wetting and drying (AWD), or permanently as in nonflooded rice production (Bouman et al. 2007; Arao et al. 2009; Li et al. 2009b; Seyfferth et al. 2018). When soils are drier (e.g. nonflooded conditions), their Eh is higher and sulfide-bound contaminants such as cadmium (Cd) can be released through oxidation of reduced minerals, resulting in plant uptake of the Cd2+ ion (Kashem and Singh 2001a, b; Arao et al. 2009; Seyfferth et al. 2019a). This dynamic leads to a tradeoff between As and Cd availability to rice during water management and efforts are currently underway to determine the most promising irrigation strategy to minimize both As and Cd in rice. We posit that a combination of water and Si management may be better than either alone in minimizing uptake of toxic metal(loid)s in rice.
Both Si and water management have the potential to affect the formation and mineral composition of Fe plaque that forms in the rhizosphere of rice, which has consequences for plant-uptake of toxic metal(loid)s. Fe plaque is apparent as the reddish-orange precipitate on the roots of wetland plants like rice grown under flooded conditions. This plaque forms due to radial oxygen loss (ROL) from aerenchyma tissues that transport oxygen from the atmosphere to roots and allow the roots to respire, but in the process some oxygen leaks into the rhizosphere and reacts with aqueous ferrous iron (Hansel et al. 2001; Cornell and Schwertmann 2006a; Khan et al. 2016). Fe plaque is heterogeneous in space and is comprised mostly of the Fe (hydr)oxides ferrihydrite (“Fh,” Fe(OH)3·nH2O), lepidocrocite (“Lep,” γ-FeOOH), and goethite (“Goe,” α-FeOOH) (Hansel et al. 2001; Cornell and Schwertmann 2006a; Seyfferth et al. 2010, 2011; Frommer et al. 2011; Khan et al. 2016). These minerals are in a dynamic equilibrium, with the short-range ordered and highly reactive Fh generally forming first and transforming into the more highly-ordered and stable Lep and Goe over time (Waychunas et al. 1993; Manceau and Charlet 1994; Manceau 1995; Schwertmann et al. 1999; Hansel et al. 2005; Cornell and Schwertmann 2006b). Fe oxides are usually positively charged in soils (Cornell and Schwertmann 2006c) and therefore strongly adsorb anions, including As species (Inskeep et al. 2002; Bogdan and Schenk 2008). It is thought that Fe plaque limits uptake of As (Hansel et al. 2002; Guo et al. 2007; Deng et al. 2010; Lee et al. 2013; Syu et al. 2013; Yamaguchi et al. 2014; Amaral et al. 2017; Yang et al. 2020), but this complex process is not well understood. It is known that Si addition retards the transformation of Fh to higher ordered phases (Schwertmann and Thalmann 1976; Amaral et al. 2017; Limmer et al. 2018a), which may effectively increase the surface area for As adsorption on plaque (Cornell and Schwertmann 2006c). However, Si can also compete with As(III) for adsorption sites (Luxton et al. 2008). In addition, less soil flooding tends to decrease the amount of Fe plaque and promote a plaque that has higher siderite (FeCO3) and Lep (Seyfferth et al. 2019b).
Because of these complex rhizosphere interactions, we must better understand how soil management impacts the uptake of nutrients and contaminants by rice. It is unknown how return of Si-rich rice husk with and without charring pretreatment will affect Si and As uptake by rice. It is also unknown how water management will affect the trade-off between As and Cd uptake in this context, and how both these management decisions will impact rhizosphere interactions. Here, we conducted a pot study to better understand how Si and water management affect As and Cd uptake by rice. We hypothesized that; 1) husk and biochar would increase plant Si and root plaque Fh content; 2) increased Si and root plaque Fh would be correlated with decreased As uptake; 3) rice As and Cd uptake would trade-off due to water management; 4) a combination of AWD water management and husk or biochar amendment would simultaneously minimize As and Cd in rice grain.
Materials and methods
Soil description and husk amendments
Soil for the pot study was collected from the University of Delaware adjacent to the Rice Investigation, Communication, and Education (RICE) Facility. Detailed soil descriptions can be found in earlier work (Teasley et al. 2017; Limmer et al. 2018a; Seyfferth et al. 2019a) but briefly, the soil is an Elsinboro series, Typic Hapludult, silty loam and has pH (1:1 H2O) = 5.2, 2.86% organic matter (loss-on-ignition), 3.8 mg kg−1 total As (acid-digestible), < 1 mg kg−1 total Cd (acid-digestible), and 13 mg kg−1 acetic acid-extractable Si. Soil was collected from the A horizons (approximately 20 cm depth) and mixed by hand while moist, taking care to preserve soil structure as closely as possible.
Rice husk treatments consisted of Control (no amendment), unaltered rice husk (“Husk”), rice husk biochar (“Biochar”), and husk incinerated at high (> 1000 °C) temperature (“CharSil”). Both Husk and CharSil were acquired from Riceland Mills in Stuttgart, Arkansas, USA. Rice husk biochar was pyrolyzed from the fresh husk in steel cannisters with a small vent in their lid via batch method at 450 °C. Cannisters were removed from the oven and sealed when volatilization ceased (39 min). Additional information on Biochar preparation and characterization can be found in Linam et al. 2021. Elemental composition of amendments and pH, cation exchange capacity (CEC), specific surface area (SSA), and point of zero charge (PZC) of Biochar and CharSil are given in Table 1.
Pot study design
A pot study was conducted to determine the effects of husk amendments and water management on Si, As, and Cd uptake by rice plants. A 4 × 3 completely randomized experimental design was employed with 4 amendment treatments and 3 water management treatments, each in triplicate. Holes were drilled into the base of 36 8 L HDPE pots, and 9.36 kg of moist soil (equivalent to 8 kg dry soil) was added to each. To each pot, 45 g (0.5% w/w) of rice straw containing 30 g kg−1 Si and 2.1 mg kg−1 As from the RICE Facility was added and mixed into the soil, the pot drainage holes were sealed with rubber stoppers, and the pots were flooded with distilled H2O. The pots were randomized and placed in a climate-controlled growth chamber for 31 days to allow equilibration of paddy-like conditions. The growth chamber was set to 14 h days with daytime temperatures of 28 °C and nighttime temperatures of 26 °C at 60% relative humidity. Light was supplied by LED LumiGro LumiBars. The plugs were then removed to allow the pots to drain before incorporating Husk, Biochar, and CharSil in 9 pots each at ~ 1 Mg Si ha−1 rates (i.e. 45 g Husk, 18.45 g Biochar, and 17.35 g Charsil per pot) while the remaining 9 pots received no amendment (“Control”). Amendments were not ground and were incorporated well into the soil to prevent loss from floating or leaching. This level of amendment is equivalent to ~ 4 years’ worth of husk production. Soil test recommended levels of nitrogen and potassium as urea and KCl (background P levels were sufficient for rice growth) were also added. Hybrid rice seedlings (Oryza sativa L. cv CLXL745) were germinated separately in a greenhouse for 24 days and were transplanted (one seedling per pot) 7 days after addition of amendments and fertilizer.
The water management treatments were imposed after transplanting seedlings into the 8 L pots. Three pots from each treatment were inundated continuously (“Flooded”), 3 were inundated but allowed to drain 4 times during vegetative growth to 25% volumetric water content which took about 7 days for our soil (alternate wetting and drying, “AWD”), and 3 were watered twice-weekly to 80% water-filled pore space then allowed to drain into a basin from which water could wick back into the soil (“Nonflooded”). Pot order was randomized weekly, and plants were grown in the growth chamber until physiological maturity (124 days post-transplant).
Porewater sampling and analysis
Soil porewater was sampled every ~ 14 days using Rhizon samplers (Rhizosphere Research Products, 19.21.01) inserted at a 45° angle into the soil (2–9 cm sampling depth) according to published methods (Seyfferth and Fendorf 2012; Seyfferth et al. 2016). These Rhizons were left in the soil, and evacuated HDPE and evacuated/combusted glass vials were used to collect porewater from each pot. Samples from glass vials were used for dissolved organic carbon (DOC) analysis (Vario TOC Cube, Elementar). The samples in plastic vials were used for pH (calibrated probe), Eh (Pt electrode calibrated against the standard hydrogen electrode), and H4SiO4 (Kraska and Breitenbeck 2010) and Fe(II) (Stookey 1970) via colorimetric determination, and the remainder was acidified (2% HNO3) and elemental composition was determined via ICP-MS (Thermo Scientific™ iCAP™ TQ ICP-MS) with matrix-matched standards. Time-weighted average values were calculated by summing the products of the measured values and their interval between sampling dates, all divided by experiment duration.
Plant analysis
After 124 days of growth post-transplant, the plants were harvested and separated into panicles, straw (stems and leaves), and roots. Roots circling the edge of the pots (“pot-bound”) were removed and not included in analysis. Panicles were air-dried, straw was dried at 40 °C, roots were washed and air-dried, and then all were weighed to obtain biomass. The grain was removed from the husk via a benchtop de-husker, then the brown grain, husk, and straw were ground into powders. Roots were processed according to the method of Amaral et al (2017) to obtain Fe plaque mineral composition. For this, approximately half of the dried roots were used for Fe plaque mineral composition (see next section) and the remaining half was subjected to a dithionite-citrate-bicarbonate (DCB) extraction to dissolve Fe plaque, and the extract was diluted 100-fold prior to elemental analysis with ICP-OES. The plaque-free roots were then washed, dried, and ground. The root, straw, husk, and grain powders were digested in concentrated HNO3 in Teflon vessels using a microwave digester (MARS6 Xpress, CEM Corporation) along with NIST 1568a (rice flour), 1570a (spinach leaves), 1573a (tomato leaves), and WEPAL IPE 188 (oil palm leaves) as standards following previously established procedures (Seyfferth et al. 2016). These digests were diluted to 2% HNO3 and elemental analysis was performed along with blanks and check standards via ICP-MS. Grain As speciation in brown rice grain was also determined via extraction with dilute HNO3, and analysis via HPLC-ICP-MS with a PRP-X100 anion exchange column (2.1 × 50 mm, Phenomenex) and Thermo Scientific™ iCAP™ TQ ICP-MS. Detailed descriptions of these procedures can be found (Kirby et al. 2004; Maher et al. 2013; Jackson 2015; Limmer et al. 2018a). Recoveries of porewater As, Cd, and Si from check standards were 102.0 ± 12.4%, 99.1 ± 2.5%, and 96.8 ± 7.5%, respectively; recoveries of As and Cd from standard reference material digests were 143 ± 57% and 114 ± 49% (NIST 1568b), 98 ± 18% and 94 ± 4% (NIST 1570a), and 95 ± 29% and 95 ± 4% (NIST 1573a), respectively. While there is not a certified reference for Si in plant tissue, the values recovered were within the range of the indicative values with an average of 71 ± 5%.
Fe mineral composition of root Fe plaque
Iron mineral composition of the root plaque was determined via Fe EXAFS. Half of the dried roots from each plant was sonicated to remove the root plaque, which was collected and dried on a nitrocellulose filter paper following previous techniques (Amaral et al. 2017). These filters were analyzed on beamline 6-BM (BMM) at NSLS-II. The samples were analyzed in duplicate using a ~ 5 × 1 mm beam in transmission out to a k of 15 Å−1 with steps of 0.05 k and integration times of 1 s. Data analysis (background subtraction, normalization, and linear combination fitting) was performed using Athena software (Ravel and Newville 2005). Standards for fitting were constrained by x-ray diffraction results from previous studies of root plaque (Hansel et al. 2001; Seyfferth et al. 2011; Seyfferth 2015; Amaral et al. 2017) and included 2-line Fh, Lep, Goe, siderite, hematite (Fe2O3), and magnetite (Fe3O4). Fitting was performed on k3-weighted spectra from k = 2–12 Å−1.
Grain As XRF images and associated As XANES
X-ray fluorescence (XRF) microscopy was used to elucidate the As distribution and speciation in rice grains from each treatment. Single brown grains were embedded in epoxy resin (EPO-TEK 301-2FL) and cut perpendicular to the long axis. Cross Sects. (30 μm) were fixed on quartz slides. Grains of interest were selected that had representative As concentrations and the presence of the ovular vascular trace (OVT) in the cross section. Imaging of As distribution and As XANES spot analysis was performed on XFM beamline (4-BM) at the National Synchrotron Light Source-II (NSLS-II). Briefly, this beamline uses Kirkpatrick-Baez (KB) mirrors to deliver focused X-rays (2—10 mm spot size) with tunable energy using a Si(111) double-crystal monochromator. Samples were oriented 45° to the incident beam and the XRF detector (Canberra SXD 7-element SDD) was positioned 90° to the incident beam. Coarse images were taken of entire grains at 12 keV with 10 μm beam size, 10 μm step size, and 100 ms dwell time. Fine images were taken of the OVT region at 12 keV with 3 μm beam size, 3 μm step size, and 500 ms dwell time. Images were processed using LARCH (Newville 2013) and analyzed using SMAK (Webb 2011). Arsenic K-edge micro-XANES were collected in fluorescence mode over an energy range of 11,802–12,017 eV with 3 s integration time. The peak of the first derivative was set to 11,874 eV for an As(V) standard. Transects were measured across the endosperm, bran, and OVT boundaries. Triplicate scans were averaged, normalized, and fit using linear combination fitting in Athena (Ravel and Newville 2005). Standards considered for fitting were As(III), As(V), DMA(V), MMA(V), and As(III)-Glutathione (AsGlu).
Statistics
Statistical analyses were performed using R Studio (Team 2020) and JMP Pro 16 (SAS Institute). Linear regression analysis was used to examine correlations between variables within and across treatments. Amendment*Water Management interactions were considered during two-way ANOVA and Tukey post-hoc HSD analysis. Significant differences were considered at p < 0.05 level.
Results
Porewater Si, As, and Cd
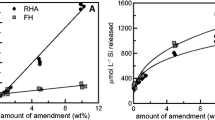
Average concentrations of Si in the soil porewater were affected by the amendment (p < 0.001), water management (p = 0.041), and amendment*water management interaction term (p < 0.001). Husk, Biochar, and CharSil amendments increased time-weighted average porewater Si concentrations over Control by 45%, 11%, and 4%, respectively, while Flooded and AWD treatments had 38% and 26% higher porewater Si compared to Nonflooded, respectively (Fig. 1a).
Time-weighted average concentrations of Si (a), As (b), and Cd (c) in soil porewater for the duration of the experiment (10 sampling dates over 124 days). Water management determined As and Cd, while amendment and water management influenced Si concentration. Uppercase letters with brackets represent Tukey HSD groupings for water management effects and uppercase letters inside bars for amendment effects. Error bars represent ± standard deviation (n = 3); α = 0.05
Concentrations of As and Cd in the soil porewater were controlled solely by water management (Fig. 1b, 1c) with no significant differences due to amendments (p = 0.1 and 0.9, respectively). Porewater As (p < 0.001) was decreased by 67% and porewater Cd (p < 0.001) was increased by 184% in Nonflooded compared to Flooded treatments; there were not significant differences in porewater As and Cd between AWD and Flooded conditions. The difference in Eh between Nonflooded and Flooded treatments also increased as plants grew larger and had increased evapotranspiration rates (Figure S2). Thus, As and Cd solubilities were inversely correlated and controlled by water management. No evidence of immobilization of As or Cd by amendments was observed.
Plant Si and yield
Porewater Si concentrations were correlated to plant Si concentrations for husk (p = 0.003), straw (p < 0.001), and roots (p = 0.010) at harvest (Table S1). Plaque Si concentration was not strongly correlated to porewater Si and was best predicted by Eh (p = 0.051). Eh was also a significant predictor in multiple linear regression models of straw (p = 0.010) and root (p = 0.004) Si concentration, where Eh was positively correlated to root Si and negatively correlated to straw Si. Though not significant, Eh was also negatively correlated to husk Si. It should be noted that the variance explained by these models was still quite low (R2adj from 0.09–0.66).
Due to the strong correlation between porewater and plant Si, amendments generally increased plant Si concentration (Fig. 2a,b). Amendments significantly (p < 0.001) impacted Si concentration in straw and roots; water management significantly impacted straw (p < 0.001), root (p = 0.010), and plaque (p = 0.044) Si; and water management*amendment interaction significantly affected husk Si (p = 0.009). Increased flooding tended to increase straw Si while decreasing root Si (Fig. 2, Table S1).
Concentrations of Si in aboveground (a) and belowground (b) plant tissues at harvest determined via plant digestion; aboveground values are in thousands. In legend, “C” = Control, “H” = Husk, “BC” = Biochar, “CH” = CharSil, and “NF” = Nonflooded, “AWD” = Alternate Wetting and Drying, “F” = Flooded. Amendment is seen to affect straw and root Si (Tukey HSD groupings in capital letters with brackets), water management affects straw, root, and plaque Si (capital letters above error bars), and water management*amendment interaction affects husk Si (lowercase letters above error bars). Error bars represent ± standard deviation (n = 3); α = 0.05
Patterns of grain and straw yield differed slightly among treatments, with AWD producing more grain (p = 0.01) and straw (p = 0.05) than Nonflooded water management (Fig. S1a,b). There were no significant differences in harvest index (Fig. S1c). Because concentration and total mass of As and Cd in plant tissues showed similar trends, we will present elemental concentrations rather than elemental mass in each plant fraction.
Concentrations and tradeoff of grain As and Cd
Rice grain total As and Cd concentration was largely controlled by water management (Fig. 3, p < 0.001 for both), which was the only statistically significant independent variable. Flooded rice accumulated 1.3–2.3 times more As than AWD, and 9–12 times more than Nonflooded. Conversely, Nonflooded rice accumulated 7.3–16.8 times more Cd than AWD, and 16.7–40.3 times more than Flooded. AWD treatment effectively increased average porewater Eh by 51–168 mV during the 30 days of dry downs and maintained a higher Eh for several weeks after re-flooding (Figure S2). Husk and Biochar treatments showed a smaller increase in Eh compared to Control and CharSil. Increased As uptake due to flooding generally increased up the transpiration stream (from plaque to grain), while increased Cd uptake due to Nonflooded conditions was most pronounced in plaque and grain. Amendments did not significantly impact plant Cd concentrations. Unpolished rice grain As and Cd concentrations were exponentially correlated to porewater Eh, and inversely correlated with each other. This results in a trade-off between grain As and Cd, with minimization of both contaminants at moderate Eh of 225–275 mV (Fig. 4).
Concentrations of As species (a) and total Cd (b) in brown grain at harvest determined via microwave digestion. TMAO = trimethylarsine oxide. Total As, DMA, and total Cd are all significantly affected by water management (capital letters with brackets), while iAs concentration is affected by water management*amendment interaction (lowercase letters inside bars). Error bars represent ± standard deviation (n = 3); α = 0.05
Exponential trade-off between grain As (circles) and Cd (squares) showing minimization of both contaminants at Eh of 225–275 mV. This value is expected to shift in different soils with different As and Cd levels. Time-weighted average porewater Eh is shown to be a good sole predictor of grain contamination (R2 = 0.7–0.75)
Concentrations of Cd in plant tissues (roots, straw, husk, and grain) could be predicted very well by porewater Eh and porewater Cd concentration (Table S1). In contrast, the dynamics of As uptake and translocation were more complex (Table S1, Fig. S3). Generally, plant As decreased with increasing Eh. When all samples are analyzed together, there are no clear correlations between porewater Si and plant As; Eh alone was an equally good predictor of grain As when compared to more complex models. When samples are separated by water management, however, we see reasonably strong negative correlations between porewater Si and As in husk, straw, roots, and grain (for iAs; DMA shows no such trend, Fig. S3).
Arsenic speciation in the rice grain was much more variable than total As concentration (Fig. 3a). Concentrations of organic As species (oAs, primarily DMA) in rice grain was only significantly impacted by water management, and the trend was the same as total As: F > AWD > NF. Inorganic As (iAs = As(III) + As(V)) was significantly affected by amendment (p = 0.026), water management (p < 0.001), and the interaction term of these two variables (p = 0.009; Fig. 3a). Under Flooded treatments, the Control, Biochar and CharSil treatments had the highest grain iAs, which was slightly lowered, but not significantly, in the Husk treatment. Under AWD, the lowest grain iAs was observed in the Control with Biochar treatment having significantly more iAs. Between Flooded and AWD water managements, the largest impact observed was that AWD decreased grain iAs by 65% in the Control, while the Husk, Biochar, and CharSil treatments only showed a 5–22% decrease in iAs (Fig. 3a). AWD decreased oAs species by 44–81% and showed no trends due to amendment.
Arsenic μXRF maps of rice grains provided additional insight into the changes of As speciation under Flooded (Fig. 5b) to AWD (Fig. 5a) conditions (example shown from Husk amendments). The ovular vascular trace (OVT) appears more intense in the AWD grain (Fig. 5a) due to iAs being the primary species under AWD (Fig. 3a) and being concentrated more in the OVT. The OVT of the Flooded grain (Fig. 5b) appears similar or even less pronounced, which suggests the decreased grain As due to AWD seen in the digestion data (Fig. 3a) is primarily due to less DMA distributed in the bran and endosperm. Arsenic μ-XANES spectra were collected from spots shown in Fig. 5 and are classified as endosperm, bran, or OVT based on their location. Results from μ-XANES support the evidence of AWD primarily decreasing grain DMA, not iAs (Fig. 6), and also suggest that the majority of the “iAs” seen via HPLC-ICP-MS is likely As(III)-glutathione (AsGlu) that is oxidized during grain extraction (Raab et al. 2004; Lombi et al. 2009). As-XANES data showed an increase in AsGlu dominance due to AWD water management, the magnitude of which decreases moving into the grain (OVT > bran > endosperm; Fig. 6). As-XANES data from Biochar and CharSil AWD grains showed a similar but stronger trend of increased AsGlu and As(V) with decreased DMA under AWD water management (Fig. S4). Finally, example As μXRF maps of grain cross-sections for CharSil-Flooded (Fig. 7b,e) and CharSil-AWD (Fig. 7a,d) shows evidence of much lower As in the bran and endosperm of AWD grains, with the unchanged OVT signal, likely due to iAs.
μXRF maps showing total As distribution in the grain OVT region of rice grown under AWD (a) and Flooded (b) water management with Husk treatment. Markers indicate location of As XANES scans along with labels corresponding to their location in the grain. Color scales are set to the same minimum and maximum fluorescence intensity
Bar charts showing linear combination fitting results of As XANES spot scans in rice grain from AWD and Flooded experiments under Husk treatment. Tissue locations correspond to labels in Fig. 5. AWD treatment was seen to generally decrease the DMA proportion of total As. Magnitude of trend is highest in OVT and bran, and trend is similar in Biochar and CharSil treatments (Fig. S4)
μXRF images of As distribution in grains from rice grown under AWD (a,c) and Flooded (b,e) water management with CharSil treatment. Lower images (c and e) are fine scans of OVT regions delineated by white boxes in a and b, respectively. Under Flooded conditions, As is much more diffuse throughout the endosperm due to higher DMA, while it is concentrated as arsenic(III)-glutathione (”iAs” in extraction data) in the bran and OVT in AWD treatment. This corresponds to the As speciation data from the grain extraction data (d, copied from Fig. 3a) which suggests AWD treatment primarily decreases DMA which is located throughout the endosperm. Color scales are set to the same minimum and maximum fluorescence intensities for the coarse scans (a,b) and for the fine scans (c, e)
Plaque Si, As, and mineralogy
Root plaque mineral composition was impacted by both water management (p < 0.001) and water management*amendment interaction (p = 0.023, Fig. 8). Linear combination fitting results suggested minimization of error with a 4-component model consisting of 2-line Fh, Lep, Goe, and siderite. Total amount of plaque decreased from Flooded > AWD > Nonflooded, while the amount of Fh and Lep is influenced by water management*amendment interaction (p = 0.023 and 0.036, respectively). Although there is significantly less Lep and noticeably less Fh, Goe, and siderite in the Flooded Husk treatment compared to the Flooded Control, this relationship is inverted for the AWD treatment. On a percentage basis, multiple linear regression suggests that percent Fh increases with Eh; percent Goe increases and percent siderite decreases with Fe(II); and percent Lep decreases with Fe(II), DOC, and Eh. There is a significant impact of water management on Fe mineralogy on a percent basis, where Nonflooded conditions increase Fh and Lep at the expense of Goe (Fig. S5). On a mass basis (g Fe mineral/kg root), multiple linear regression shows that the mass of each mineral decreases with Eh, and that Lep decreases with DOC. Porewater DOC and Si are positively correlated to Fh, though they are not statistically significant predictors.
Concentration of Fe minerals in root plaque determined via Fe-EXAFS and DCB extraction showing overall Fe plaque increased with flooding extent. Uppercase letters with brackets represent Tukey HSD groupings for water management and total, Sid, Goe, Lep, and Fh mass in plaque. Lowercase letters in bars represent Tukey HSD groupings within Fe minerals for water management*amendment interaction. Error bars represent standard deviation (n = 3); α = 0.05
Linear regression shows an inverse relationship between As translocation factor (TF = grain As/porewater As) and percent Fh in root plaque, while As translocation is positively correlated to plaque percent Goe (Fig. 9). Despite these correlations, grain As is closely tied to total amount of Fe plaque on roots, as emphasized by the similar trends seen in Figs. 3a and 8. Figure 9 avoids biases due to Eh effects and thus focuses on relative As accumulation from porewater.
Positive and inverse linear correlations between As translocation factor (TF) and percent Fe plaque as Goe and Fh, respectively. As TF = grain As/time-weighted average porewater As. Slopes suggest that a 10% increase in plaque Goe results in 1.48 mg/kg more As translocated to grain per μM porewater As, while 10% more Fh in plaque results in 2.13 mg/kg less As translocated to grain per porewater μM As
Discussion
Si supply from amendments
We partially accept hypothesis 1—Husk and Biochar increased plant Si but did not seem to increase Fh in root plaque. Rice husk management plays a strong role in determining plant available Si. Soil incorporation of Husk and Biochar increases Si concentrations in porewater and rice plants regardless of water management (Figs. 1,2). This agrees with the literature (Penido et al. 2016; Seyfferth et al. 2016), and suggests Si-rich residue incorporation plays an important role in the Si cycle in rice paddies (Seyfferth et al. 2013). Uncontrolled combustion of husk at high temperature, however, inhibits the ability of husk to supply Si to soil or plants; for CharSil, this is likely due to the transformation of amorphous SiO2 to more crystalline forms (Savant et al. 1997). Typical pyrolytic temperatures do not cause crystallinity changes of SiO2, and therefore the decreased Si supply from Biochar seen here is likely due to increased hydrophobicity or resistance to decomposition (Linam et al. 2021). In contrast to previous work (Taib 2007; Xiao et al. 2014), our results suggest that the ability of rice husk to supply plant-available Si is not increased by pyrolysis or burning pretreatment and may actually be decreased by it. It is also worth noting that porewater (Fig. 1) and aboveground plant (Fig. 2) Si increased with extent of flooding (Tsujimoto et al. 2014). This is likely due to release of Si bound to minerals such as Fe (hydr)oxides during reductive dissolution at low Eh. This has important implications for the role root or rhizosphere Fe plaque could play in Si availability to rice.
Rice As uptake and grain speciation
Uptake of As by rice plants is a complicated process, but our results suggest it is primarily determined by soil redox potential (Fig. 4, Table S1). There is a strong exponential relationship between grain As and porewater Eh as previously observed (Li et al. 2009b), which is not improved by considering porewater As (Seyfferth et al. 2019a); however, here porewater As was positively correlated with husk and straw As (Table S1). This could be due to active biological control over As translocation to rice grain, differences in Si:As ratios, high levels of DMA in grain, or strong collinearity between porewater Eh and As.
Amendments interacted with water management to alter As speciation in rice grain. This was particularly important in AWD water management, where (compared to Flooded) AWD mainly decreased grain DMA regardless of amendment and only decreased iAs in Control (Fig. 3a). Our results suggest that AWD is more effective at decreasing rice grain DMA compared to iAs in accordance with some previous work (Li et al. 2009b; Ma et al. 2014) and that amendments may further this effect. This suggests a lower Eh is necessary for As methylation compared to iAs reduction (Chen et al. 2019)—methylation can similarly be promoted by addition of labile carbon (Jia et al. 2013; Yang et al. 2018). Other studies suggest no trend (Chou et al. 2016; Das et al. 2016) in the effects of AWD on As speciation; this is likely due to soil-specific responses to AWD which could also help explain the high variability in As speciation seen worldwide (Zhao et al. 2013). Within the AWD treatment, amendments actually led to higher iAs concentrations than Control (Fig. 3a). The grain digestion results are supported by the As-XANES spectra, which show AsGlu dominates As speciation under AWD, particularly in the OVT and bran (Figs. 6,S3). The As μXRF maps also show decreased As presence in the grain interior due to AWD (Fig. 7), which supports lower DMA concentration, as DMA is generally found more in the endosperm compared to iAs or AsGlu (Lombi et al. 2009; Carey et al. 2010; Zheng et al. 2013; Limmer et al. 2018a). However, we only have direct comparisons between Flooded and AWD grain for Husk treatment, so it is unclear how much of the iAs in AWD Control might be comprised of iAs(III) rather than As(III)-glutathione. It is thought that As(III)-glutathione has similar toxicity to iAs due to disassociation of the complex during digestion (Zakharyan and Aposhian 1999), and thus decreasing its concentration in grain is equally important. Because these species are likely more toxic to humans than DMA (Kenyon and Hughes 2001), the effectiveness of AWD requires consideration of As speciation. Therefore, AWD treatments (in this soil type at least) should include more or longer dry downs to decrease iAs in rice grain when rice husks (untreated or charred) are returned to the soil.
Si-As interaction
Our data supports hypothesis 2—we see decreased husk and straw As and grain iAs (for Flooded water management) with higher porewater Si. We also see a negative correlation between plaque Fh concentration and root As and As translocation, but evidence is lacking that our low levels of amendments increased plaque Fh. This study demonstrated that Husk and Biochar are effective amendments to increase Si concentration in porewater and in aboveground rice tissues (Figs. 1,2). In accordance with what has been proposed by molecular and agricultural research (Guo et al. 2005; Jian et al. 2006; Ma et al. 2007, 2008; Bogdan and Schenk 2008; Zhao et al. 2009; Li et al. 2009a; Seyfferth and Fendorf 2012; Seyfferth et al. 2018, 2019a, 2016; Limmer et al. 2018b, a), Si in porewater was negatively correlated with As concentration in rice straw and husk (Table S1, Fig. S3). This antagonistic relationship was not seen with grain As across treatments in multiple linear regression (Table S1), but under Flooded conditions, porewater Si was negatively (but weakly) correlated with grain iAs (Fig. S3). This suggests the Si-As uptake competition affects grain As differently under different water managements. While previous thought suggests that Si promotes DMA formation and uptake relative to iAs (Seyfferth et al. 2018), our data show that Si-rich amendments decrease DMA relative to iAs under AWD management (Fig. 3). We attribute this to decreased Eh due to the labile carbon present in our amendments. The low, background As levels in our soil might be less affected by Si addition compared to high or spiked soil As from other studies (Seyfferth and Fendorf 2012; Teasley et al. 2017).
It is also possible that the Ultisol used in this study is still Si-deficient even after amendment with ~ 4-years’ worth of rice husk and/or the cultivar used here has a higher demand for Si. This is supported by the relatively low concentrations of Si in the rice straw (< 3%) and husk (< 4%) at harvest (Fig. 2) and generally low porewater Si concentrations even with amendments (Fig. 1a). A mild Si-deficiency could explain why Si decreased As uptake into vegetative tissues but not grain—Si has been seen to affect vegetative tissue As more than grain As (Li et al. 2009b; Seyfferth and Fendorf 2012; Limmer et al. 2018a; Seyfferth et al. 2018). Rice grain also accumulates a higher proportion of As in DMA form compared to vegetative tissues, and porewater Si competes best with iAs (Seyfferth et al. 2016, 2018), which could help explain the stronger correlation between porewater Si and vegetative As compared to grain As (Fig. S3). Additional measures besides return of husk to highly weathered paddy soils may be necessary to gain the As-limiting benefits of Si fertilization (Klotzbücher et al. 2015; Hughes et al. 2020). We therefore reject hypothesis 4—the competition between Si and grain As uptake is only seen under Flooded conditions and does not seem to be additive to the As-limiting effects of AWD.
Rice As and Cd management
Our data fully supports hypothesis 3—there is a clear tradeoff between grain As and Cd due to soil Eh which can be manipulated using AWD. Co-contamination of rice grain by Cd and As was minimized in the Eh range of 225–275 mV according to exponential fits (Fig. 4). Few of our treatments were in this Eh range, however, suggesting that in order to achieve this minimization, more severe and additional dry downs are required for the AWD treatment. This value is also very relative, and is expected to be different for every soil (Rinklebe et al. 2016), making management challenging. Other studies report “optimal” Eh values which differ appreciably from that reported here (Honma et al. 2016; Yao et al. 2021), and emphasize that optimal redox conditions are dependent on soil type, poise, and As and Cd levels in addition to redox measurement method, timing, and accuracy. It is likely that other measurements are also calibrated to different reference electrodes than the standard hydrogen electrode, which adds to the discrepancy. Due to the dynamic tradeoff between grain As and Cd, water management in paddy soils must consider the relative levels of contaminants, in addition to amendments which were seen to decrease the efficiency of dry downs for minimizing iAs here by threefold for Husk and CharSil and 14-fold for Biochar (Fig. 3a). This result is likely due to the balance of soluble Si (which decreases iAs uptake) and labile carbon (which decreases Eh and increases As mobility) in our amendments. Husk and Biochar maintained a lower Eh under AWD (Fig. S2), but our results suggest the more rapid dissolution of Si from Husk was able to counteract the decreased Eh better than the slower dissolution of Si from Biochar.
There were no significant effects of amendments on plant or porewater Cd concentrations in this study which suggests that pyrolyzing or charring rice husk does not make it a significant adsorbent or immobilization agent for soil Cd. Although isotherms measured in lab settings show significant Cd adsorption, Cd2+ is a soft acid cation which likely cannot compete for the hard base CEC sites which are dominant on rice husk biochar (Linam et al. 2021). It is also likely that the active surface of the Biochar was fouled by organic matter, clays, or microbes upon incorporation into soil. Because our amendment rates were relatively low, Biochar and CharSil were not able to raise the porewater pH (Fig. S2) and therefore failed to immobilize the Cd via precipitation reactions. Cadmium availability is generally high in weathered and acidic soil with low OM and CEC (Sauvé et al. 2000; Shaheen et al. 2017; Jalali and Najafi 2018; Mu et al. 2019) such as our soil; in these soil conditions, Cd availability is primarily controlled by redox potential (Arao et al. 2009; Rinklebe et al. 2016; Zhao and Wang 2020). We therefore conclude that higher amendment rates or continued return of rice husk as Biochar or CharSil would be necessary to cause Cd immobilization, likely through raising soil pH (Table 1).
Root plaque chemistry
While previous studies have proposed Fe plaque as a protective barrier for As uptake by rice (Hansel et al. 2002; Guo et al. 2007; Deng et al. 2010; Lee et al. 2013; Syu et al. 2013; Yamaguchi et al. 2014; Amaral et al. 2017; Yang et al. 2020), we see a strong correlation between root Fe plaque and grain As (Figs. 3a, 8). This would seem to suggest that roots accumulate Fe plaque and grain accumulates As due to similar causal factors—both processes seem to be most strongly controlled by Eh. Fe plaque mineralogy is seen to be important, however, where higher Fh content is correlated to lower translocation of As from porewater to grain, while higher Goe content is correlated to higher translocation of As (Fig. 9). Goe content is seen to be correlated to porewater Fe(II) and to be favored over Lep at higher DOC concentrations. While it is possible that Si and DOC stabilize Fh and discourage its transformation to more crystalline forms (Schwertmann and Thalmann 1976; Anderson and Benjamin 1985; Cornell and Schwertmann 2006b; Chen et al. 2015; Seyfferth 2015; Amaral et al. 2017; Limmer et al. 2018a), these are not significant predictors in our study, and suggest that our low levels of amendments do not increase Fh formation. This is contrary to expectations based on literature (Seyfferth 2015; Amaral et al. 2017; Limmer et al. 2018a), and suggests more complex controls on plaque formation—we attribute this discrepancy to the low Si concentration in our soil and lower amendment rates compared to previous studies which muted the effect of Si on Fh stabilization.
When viewed on a mass basis, plaque Fh does not seem to decrease aboveground plant As, but it does decrease root As (Table S1). Though all plaque mineral masses are positively correlated with plant As concentration, Fe plaque mineralogy is still an important factor determining uptake and translocation of As from porewater to grain (Fig. 9). Correlations were similar for translocation of As into straw and husk but were significantly weaker for translocation into roots. This is likely due to roots acting as an additional sink for As. We propose that plaque mineralogy and As uptake are both being affected by the same underlying factors such as soil type, plant nutritional status and cultivar, or root physiology. This would result in collinearity between plaque mineralogy and plant As, making it difficult to discern a mechanistic understanding of plaque’s protective role. Finally, although we used 8 L pots to provide ample soil volume for root development, the plants did become pot-bound at maturity. These roots were excluded from analysis, but there is a chance that this non-ideal growth pattern alters root plaque development.
Summary
We conclude that the As-inhibiting effects of Si may not be significant in all cases. Specifically, situations with Si-sufficiency, high soil As, external As input, and constant flooding may show a more antagonistic relationship between Si and As in rice. In our study, we conclude that small amounts of husk amendments (~ 4 years’ worth) are sufficient to increase porewater and plant Si and decrease straw and husk As, but insufficient to significantly decrease grain As in all water managements. The low background soil As and Si levels and suspected Si-deficiency likely contributed to these results. The increases in plant Si due to amendments were negated when husk was pretreated at high temperature (i.e., CharSil). Grain As and Cd have inverse exponential relationships with Eh which are primarily driven by water management. Dry downs in AWD treatment were ineffective at decreasing inorganic As in the presence of amendments—this could be due to Si from amendments competing with As for adsorption sites on Fe oxides in the soil or root plaque. In addition, amendments could provide labile carbon to microbes which maintain a lower Eh throughout the dry-downs–this is supported by slightly lower grain Cd in Husk and Biochar treatment under AWD compared to Control (Fig. 3b). We also conclude that even though Fh content of Fe plaque seems to decrease As translocation, the overall amount of plaque is dependent on Eh, which results in the amount of all plaque minerals being positively correlated with grain As. In summary, management of rice husk is very important for plant Si concentration, but higher amendment rates are necessary to decrease As concentrations in grain, and more intense/frequent AWD dry downs are necessary with rice husk amendments and should be further explored.
Data availability
Data is available upon request.
Abbreviations
- AWD:
-
Alternate wetting and drying
- Fh:
-
Ferrihydrite
- Lep:
-
Lepidocrocite
- Goe:
-
Goethite
References
Alexandre A, Meunier JD, Colin F, Koud JM (1997) Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochim Cosmochim Acta 61:677–682. https://doi.org/10.1016/S0016-7037(97)00001-X
Amaral DC, Lopes G, Guilherme LRG, Seyfferth AL (2017) A New Approach to Sampling Intact Fe Plaque Reveals Si-Induced Changes in Fe Mineral Composition and Shoot As in Rice. Environ Sci Technol 51:38–45. https://doi.org/10.1021/acs.est.6b03558
Anderson PR, Benjamin MM (1985) Effects of Silicon on the Crystallization and Adsorption Properties of Ferric Oxides. Environ Sci Technol 19:1048–1053. https://doi.org/10.1021/es00141a004
Arao T, Kawasaki A, Baba K et al (2009) Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ Sci Technol 43:9361–9367. https://doi.org/10.1021/es9022738
Berthelsen S, Noble AD, Kingston G, et al (2003) Improving yield and ccs in application of silicon based sugarcane through the amendments. Townsville
Bogdan K, Schenk MK (2008) Arsenic in Rice ( Oryza sativa L.) Related to Dynamics of Arsenic and Silicic Acid in Paddy Soils. Environ Sci Technol 42:1–7. https://doi.org/10.1021/es801194q
Bouman BM, Lampayan RM, Tuong TP (2007) Water Management in Irrigated Rice: Coping with Water Scarcity. International Rice Research Institute, Los Baños, Philippines
Carey AM, Norton GJ, Deacon C et al (2011) Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol 192:87–98. https://doi.org/10.1111/j.1469-8137.2011.03789.x
Carey AM, Scheckel KG, Lombi E et al (2010) Grain unloading of arsenic species in rice. Plant Physiol 152:309–319. https://doi.org/10.1104/pp.109.146126
Chauhan BS, Jabran K, Mahajan G (2017) Rice Production Worldwide. Springer International Publishing, Cham, Switzerland
Chen C, Kukkadapu R, Sparks DL (2015) Influence of Coprecipitated Organic Matter on Fe2+(aq)-Catalyzed Transformation of Ferrihydrite: Implications for Carbon Dynamics. Environ Sci Technol 49:10927–10936. https://doi.org/10.1021/acs.est.5b02448
Chen C, Li L, Huang K et al (2019) Sulfate-reducing bacteria and methanogens are involved in arsenic methylation and demethylation in paddy soils. ISME J 13:2523–2535. https://doi.org/10.1038/s41396-019-0451-7
Chou ML, Jean JS, Sun GX et al (2016) Irrigation practices on rice crop production in arsenic-rich paddy soil. Crop Sci 56:422–431. https://doi.org/10.2135/cropsci2015.04.0233
Cornelis JT, Delvaux B (2016) Soil processes drive the biological silicon feedback loop. Funct Ecol 30:1298–1310. https://doi.org/10.1111/1365-2435.12704
Cornell RM, Schwertmann U (2006a) Soils. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd edn. John Wiley & Sons Inc, New York, NY, pp 433–474
Cornell RM, Schwertmann U (2006b) Formation. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd edn. John Wiley & Sons Inc, New York, NY, pp 345–364
Cornell RM, Schwertmann U (2006c) Adsorption of Ions and Molecules. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd edn. John Wiley & Sons Inc, New York, NY, pp 253–296
Das S, Chou ML, Jean JS et al (2016) Water management impacts on arsenic behavior and rhizosphere bacterial communities and activities in a rice agro-ecosystem. Sci Total Environ 542:642–652. https://doi.org/10.1016/j.scitotenv.2015.10.122
Deng D, Wu SC, Wu FY et al (2010) Effects of root anatomy and Fe plaque on arsenic uptake by rice seedlings grown in solution culture. Environ Pollut 158:2589–2595. https://doi.org/10.1016/j.envpol.2010.05.015
Epstein E (1994) The anomaly of silicon in plant biology. Proc Natl Acad Sci U S A 91:11–17
Fraysse F, Pokrovsky OS, Schott J, Meunier JD (2006) Surface properties, solubility and dissolution kinetics of bamboo phytoliths. Geochim Cosmochim Acta 70:1939–1951. https://doi.org/10.1016/j.gca.2005.12.025
Fraysse F, Pokrovsky OS, Schott J, Meunier JD (2009) Surface chemistry and reactivity of plant phytoliths in aqueous solutions. Chem Geol 258:197–206. https://doi.org/10.1016/j.chemgeo.2008.10.003
Frommer J, Voegelin A, Dittmar J et al (2011) Biogeochemical processes and arsenic enrichment around rice roots in paddy soil: Results from micro-focused X-ray spectroscopy. Eur J Soil Sci 62:305–317. https://doi.org/10.1111/j.1365-2389.2010.01328.x
Gunnarsson I, Arnórsson S (2000) Amorphous silica solubility and the thermodynamic properties of H4SiO4° in the range of 0°to 350°C at P(sat). Geochim Cosmochim Acta 64:2295–2307. https://doi.org/10.1016/S0016-7037(99)00426-3
Guo W, Hou YL, Wang SG, Zhu YG (2005) Effect of silicate on the growth and arsenate uptake by rice (Oryza sativa L.) seedlings in solution culture. Plant Soil 272:173–181. https://doi.org/10.1007/s11104-004-4732-0
Guo W, Zhu YG, Liu WJ et al (2007) Is the effect of silicon on rice uptake of arsenate (AsV) related to internal silicon concentrations, iron plaque and phosphate nutrition? Environ Pollut 148:251–257. https://doi.org/10.1016/j.envpol.2006.10.021
Hansel CM, Benner SG, Fendorf S (2005) Competing Fe(II)-Induced Mineralization Pathways of Ferrihydrite. Environ Sci Technol 39:7147–7153
Hansel CM, Fendorf S, Sutton S, Newville M (2001) Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environ Sci Technol 35:3863–3868. https://doi.org/10.1021/es0105459
Hansel CM, La Force MJ, Fendorf S, Sutton S (2002) Spatial and temporal association of As and Fe species on aquatic plant roots. Environ Sci Technol 36:1988–1994. https://doi.org/10.1021/es015647d
Haynes RJ (2014) A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci.
Honma T, Ohba H, Kaneko-Kadokura A et al (2016) Optimal Soil Eh, pH, and Water Management for Simultaneously Minimizing Arsenic and Cadmium Concentrations in Rice Grains. Environ Sci Technol 50:4178–4185. https://doi.org/10.1021/acs.est.5b05424
Hughes HJ, Hung DT, Sauer D (2020) Silicon recycling through rice residue management does not prevent silicon depletion in paddy rice cultivation. Nutr Cycl Agroecosystems 118:75–89. https://doi.org/10.1007/s10705-020-10084-8
Inskeep WP, McDermott TR, Fendorf S (2002) Arsenic (V)/(III) Cycling in Soils and Natural Waters: Chemical and Microbiological Processes. In: Frankenberger WT (ed) Environmental Chemistry of Arsenic, 1st edn. Marcel Dekker, New York, NY, pp 183–215
Jackson BP (2015) Fast ion chromatography-ICP-QQQ for arsenic speciation. J Anal at Spectrom 30:1405–1407. https://doi.org/10.1039/c5ja00049a
Jalali M, Najafi S (2018) Effect of pH on Potentially Toxic Trace Elements (Cd, Cu, Ni, and Zn) Solubility in Two Native and Spiked Calcareous Soils: Experimental and Modeling. Commun Soil Sci Plant Anal 49:814–827. https://doi.org/10.1080/00103624.2018.1435682
Jia Y, Huang H, Zhong M et al (2013) Microbial arsenic methylation in soil and rice rhizosphere. Environ Sci Technol 47:3141–3148. https://doi.org/10.1021/es303649v
Jian FM, Tamai K, Yamaji N et al (2006) A silicon transporter in rice. Nature 440:688–691. https://doi.org/10.1038/nature04590
Jones LHP, Handreck KA (1967) Silica In Soils, Plants, and Animals. Adv Agron 19:107–149. https://doi.org/10.1016/S0065-2113(08)60734-8
Kashem MA, Singh BR (2001a) Metal availability in contaminated soils: I. Effects of flooding and organic matter on changes in Eh, pH and solubility of Cd. Ni and Zn Nutr Cycl Agroecosystems 61:247–255. https://doi.org/10.1023/A:1013762204510
Kashem MA, Singh BR (2001b) Metal availability in contaminated soils: II. Uptake of Cd, Ni and Zn in rice plants grown under flooded culture with organic matter addition. Nutr Cycl Agroecosystems 61:257–266. https://doi.org/10.1023/A:1013724521349
Kenyon EM, Hughes MF (2001) A concise review of the toxicity and carcinogenicity of dimethylarsinic acid. Toxicology 160:227–236. https://doi.org/10.1016/S0300-483X(00)00458-3
Khan N, Seshadri B, Bolan N, et al (2016) Root Iron Plaque on Wetland Plants as a Dynamic Pool of Nutrients and Contaminants. In: Advances in Agronomy. pp 1–96
Kirby J, Maher W, Ellwood M, Krikowa F (2004) Arsenic species determination in biological tissues by HPLC-ICP-MS and HPLC-HG-ICP-MS. Aust J Chem 57:957–966. https://doi.org/10.1071/CH04094
Klotzbücher T, Marxen A, Vetterlein D et al (2015) Plant-available silicon in paddy soils as a key factor for sustainable rice production in Southeast Asia. Basic Appl Ecol 16:665–673. https://doi.org/10.1016/j.baae.2014.08.002
Korndorfer GH, Lepsch I (2001) Effect of silicon on plant growth and crop yield. In: Datnoff LE, Snyder GH, Korndorfer GH (eds) Silicon in Agriculture, 1st edn. Elsevier Science B.V, Amsterdam, pp 133–147
Koyama S, Inazaki F, Minamikawa K et al (2015) Increase in soil carbon sequestration using rice husk charcoal without stimulating CH4 and N2O emissions in an Andosol paddy field in Japan. Soil Sci Plant Nutr 61:873–884. https://doi.org/10.1080/00380768.2015.1065511
Kraska JE, Breitenbeck GA (2010) Simple, Robust Method for Quantifying Silicon in Plant Tissue. Commun Soil Sci Plant Anal 41:2075–2085. https://doi.org/10.1080/00103624.2010.498537
Lee CH, Hsieh YC, Lin TH, Lee DY (2013) Iron plaque formation and its effect on arsenic uptake by different genotypes of paddy rice. Plant Soil 363:231–241. https://doi.org/10.1007/s11104-012-1308-2
Li R, Ago Y, Liu W et al (2009a) The Rice Aquaporin Lsi1 Mediates Uptake of Methylated Arsenic Species. Plant Physiol 150:2071–2080
Li RY, Stroud JL, Ma JF et al (2009b) Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ Sci Technol 43:3778–3783. https://doi.org/10.1021/es803643v
Li Z, Song Z, Parr JF, Wang H (2013) Occluded C in rice phytoliths: Implications to biogeochemical carbon sequestration. Plant Soil 370:615–623. https://doi.org/10.1007/s11104-013-1661-9
Limmer MA, Mann J, Amaral DC et al (2018a) Silicon-rich amendments in rice paddies: Effects on arsenic uptake and biogeochemistry. Sci Total Environ 624:1360–1368. https://doi.org/10.1016/j.scitotenv.2017.12.207
Limmer MA, Wise P, Dykes GE, Seyfferth AL (2018b) Silicon Decreases Dimethylarsinic Acid Concentration in Rice Grain and Mitigates Straighthead Disorder. Environ Sci Technol 52:4809–4816. https://doi.org/10.1021/acs.est.8b00300
Linam F, McCoach K, Limmer MA, Seyfferth AL (2021) Contrasting effects of rice husk pyrolysis temperature on silicon dissolution and retention of cadmium (Cd) and dimethylarsinic acid (DMA). Sci Total Environ 765:. https://doi.org/10.1016/j.scitotenv.2020.144428
Lombi E, Scheckel KG, Pallon J et al (2009) Speciation and distribution of arsenic and localization of nutrients in rice grains. New Phytol 184:193–201. https://doi.org/10.1111/j.1469-8137.2009.02912.x
Luxton TP, Eick MJ, Rimstidt DJ (2008) The role of silicate in the adsorption/desorption of arsenite on goethite. Chem Geol 252:125–135. https://doi.org/10.1016/j.chemgeo.2008.01.022
Ma JF (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50:11–18. https://doi.org/10.1080/00380768.2004.10408447
Ma JF, Takahashi E (2002) Soil, fertilizer, and plant silicon research in Japan. Elsevier B.V.
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397. https://doi.org/10.1016/j.tplants.2006.06.007
Ma JF, Yamaji N, Mitani N et al (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci 105:9931–9935. https://doi.org/10.1073/pnas.0802361105
Ma JF, Yamaji N, Mitani N et al (2007) An efflux transporter of silicon in rice. Nature 448:209–212. https://doi.org/10.1038/nature05964
Ma R, Shen J, Wu J et al (2014) Impact of agronomic practices on arsenic accumulation and speciation in rice grain. Environ Pollut 194:217–223. https://doi.org/10.1016/j.envpol.2014.08.004
Maher W, Foster S, Krikowa F et al (2013) Measurement of inorganic arsenic species in rice after nitric acid extraction by HPLC-ICPMS: Verification using XANES. Environ Sci Technol 47:5821–5827. https://doi.org/10.1021/es304299v
Manceau A (1995) The mechanism of anion adsorption on iron oxides: Evidence for the bonding of arsenate tetrahedra on free Fe(O, OH)6 edges. Geochim Cosmochim Acta 59:3647–3653. https://doi.org/10.1016/0016-7037(95)00275-5
Manceau A, Charlet L (1994) The Mechanism of Selenate Adsorption on Goethite and Hydrous Ferric Oxide. J Colloid Interface Sci 168:87–93
Minami E, Saka S (2005) Biomass resources present in Japan - Annual quantities grown, unused and wasted. Biomass Bioenerg 29:310–320. https://doi.org/10.1016/j.biombioe.2004.06.012
Mu T, Wu T, Zhou T et al (2019) Geographical variation in arsenic, cadmium, and lead of soils and rice in the major rice producing regions of China. Sci Total Environ 677:373–381. https://doi.org/10.1016/j.scitotenv.2019.04.337
N.K. Savant GHS and LED (1997) Silicon Management and Sustainable Rice Production. Adv Agron 58:151–199
Newville M (2013) Larch: An analysis package for XAFS and related spectroscopies. J Phys Conf Ser 430:. https://doi.org/10.1088/1742-6596/430/1/012007
Penido ES, Bennett AJ, Hanson TE, Seyfferth AL (2016) Biogeochemical impacts of silicon-rich rice residue incorporation into flooded soils: Implications for rice nutrition and cycling of arsenic. Plant Soil 399:75–87. https://doi.org/10.1007/s11104-015-2682-3
Raab A, Meharg AA, Jaspars M et al (2004) Arsenic-glutathione complexes - Their stability in solution and during separation by different HPLC modes. J Anal at Spectrom 19:183–190. https://doi.org/10.1039/b307945g
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12:537–541. https://doi.org/10.1107/S0909049505012719
Rinklebe J, Shaheen SM, Yu K (2016) Release of As, Ba, Cd, Cu, Pb, and Sr under pre-definite redox conditions in different rice paddy soils originating from the U.S.A. and Asia. Geoderma 270:21–32. https://doi.org/10.1016/j.geoderma.2015.10.011
Runkle BRK, Seyfferth AL, Reid MC et al (2021) Socio-Technical Changes for Sustainable Rice Production: Rice Husk Amendment, Conservation Irrigation, and System Changes. Front Agron 3:1–14. https://doi.org/10.3389/fagro.2021.741557
Sauvé S, Hendershot W, Allen HE (2000) Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ Sci Technol 34:1125–1131. https://doi.org/10.1021/es9907764
Savant NK, Datnoff LE, Snyder GH (1997) Depletion of plant-available silicon in soils: A possible cause of declining rice yields. Commun Soil Sci Plant Anal 28:1245–1252. https://doi.org/10.1080/00103629709369870
Schwertmann U, Friedl J, Stanjek H (1999) From Fe(III) ions to ferrihydrite and then to hematite. J Colloid Interface Sci 209:215–223. https://doi.org/10.1006/jcis.1998.5899
Schwertmann U, Thalmann H (1976) The Influence of [Fe(II)], [Si], and pH on the formation of lepidocrocite and ferrihydrite during oxidation of aqueous FeCl 2 solutions. Clay Miner 11:189–200. https://doi.org/10.1180/claymin.1976.011.3.02
Seck PA, Diagne A, Mohanty S, Wopereis MCS (2012) Crops that feed the world 7: Rice. Food Secur 4:7–24. https://doi.org/10.1007/s12571-012-0168-1
Seyfferth AL (2015) Abiotic effects of dissolved oxyanions on iron plaque quantity and mineral composition in a simulated rhizosphere. Plant Soil 397:43–61. https://doi.org/10.1007/s11104-015-2597-z
Seyfferth AL, Amaral D, Limmer MA, Guilherme LRG (2019a) Combined impacts of Si-rich rice residues and flooding extent on grain As and Cd in rice. Environ Int 128:301–309. https://doi.org/10.1016/j.envint.2019.04.060
Seyfferth AL, Fendorf S (2012) Silicate mineral impacts on the uptake and storage of arsenic and plant nutrients in rice (Oryza sativa L.). Environ Sci Technol 46:13176–13183. https://doi.org/10.1021/es3025337
Seyfferth AL, Kocar BD, Lee JA, Fendorf S (2013) Seasonal dynamics of dissolved silicon in a rice cropping system after straw incorporation. Geochim Cosmochim Acta 123:120–133. https://doi.org/10.1016/j.gca.2013.09.015
Seyfferth AL, Limmer M, Wu W (2019b) Si and Water Management Drives Changes in Fe and Mn Pools that Affect As Cycling and Uptake in Rice. Soil Syst 3:58. https://doi.org/10.3390/soilsystems3030058
Seyfferth AL, Limmer MA, Dykes GE (2018) On the Use of Silicon as an Agronomic Mitigation Strategy to Decrease Arsenic Uptake by Rice. In: Sparks DL (ed) Advances in Agronomy, 1st edn. Elsevier Inc., pp 49–91
Seyfferth AL, Morris AH, Gill R et al (2016) Soil Incorporation of Silica-Rich Rice Husk Decreases Inorganic Arsenic in Rice Grain. J Agric Food Chem 64:3760–3766. https://doi.org/10.1021/acs.jafc.6b01201
Seyfferth AL, Webb SM, Andrews JC, Fendorf S (2010) Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L) roots having variable Fe coatings. Environ Sci Technol 44:8108–8113. https://doi.org/10.1021/es101139z
Seyfferth AL, Webb SM, Andrews JC, Fendorf S (2011) Defining the distribution of arsenic species and plant nutrients in rice (Oryza sativa L.) from the root to the grain. Geochim Cosmochim Acta 75:6655–6671. https://doi.org/10.1016/j.gca.2011.06.029
Shaheen SM, Antoniadis V, Kwon EE et al (2017) Biosolids application affects the competitive sorption and lability of cadmium, copper, nickel, lead, and zinc in fluvial and calcareous soils. Environ Geochem Health 39:1365–1379. https://doi.org/10.1007/s10653-017-9927-4
Stookey LL (1970) Ferrozine–A New Spectrophotometric Reagent for Iron. Anal Chem 42:779–781
Struyf E, Mrth CM, Humborg C, Conley DJ (2010) An enormous amorphous silica stock in boreal wetlands. J Geophys Res Biogeosciences 115:1–8. https://doi.org/10.1029/2010JG001324
Syu CH, Jiang PY, Huang HH et al (2013) Arsenic sequestration in iron plaque and its effect on As uptake by rice plants grown in paddy soils with high contents of As, iron oxides, and organic matter. Soil Sci Plant Nutr 59:463–471. https://doi.org/10.1080/00380768.2013.784950
Taib MR Bin (2007) Production of Amorphous Silica from Rice Husk in Fluidised Bed System
Team RC (2020) R: A language and environment for statistical computing
Teasley WA, Limmer MA, Seyfferth AL (2017) How Rice (Oryza sativa L.) Responds to Elevated As under Different Si-Rich Soil Amendments. Environ Sci Technol 51:10335–10343. https://doi.org/10.1021/acs.est.7b01740
Tsujimoto Y, Muranaka S, Saito K, Asai H (2014) Limited Si-nutrient status of rice plants in relation to plant-available Si of soils, nitrogen fertilizer application, and rice-growing environments across Sub-Saharan Africa. F Crop Res 155:1–9. https://doi.org/10.1016/j.fcr.2013.10.003
Voogt W, Sonneveld C (2001) Silicon in Agriculture
Waychunas GA, Rea BA, Fuller CC, Davis JA (1993) Surface chemistry of ferrihydrite: Part 1. EXAFS studies of the geometry of coprecipitated and adsorbed arsenate. Geochim Cosmochim Acta 57:2251–2269. https://doi.org/10.1016/0016-7037(93)90567-G
Webb SM (2011) The MicroAnalysis Toolkit: X-ray Fluorescence Image Processing Software. In: Amer. Inst. Phys. Conf. Proc.
Wu W, Limmer MA, Seyfferth AL (2020) Quantitative assessment of plant-available silicon extraction methods in rice paddy soils under different management. Soil Sci Soc Am J 84:618–626. https://doi.org/10.1002/saj2.20013
Xiao X, Chen B, Zhu L (2014) Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures. Environ Sci Technol 48:3411–3419. https://doi.org/10.1021/es405676h
Yamaguchi N, Ohkura T, Takahashi Y et al (2014) Arsenic distribution and speciation near rice roots influenced by iron plaques and redox conditions of the soil matrix. Environ Sci Technol 48:1549–1556. https://doi.org/10.1021/es402739a
Yang Y, Hu H, Fu Q, et al (2020) Phosphorus regulates As uptake by rice via releasing As into soil porewater and sequestrating it on Fe plaque. Sci Total Environ 738:. https://doi.org/10.1016/j.scitotenv.2020.139869
Yang YP, Zhang HM, Yuan HY et al (2018) Microbe mediated arsenic release from iron minerals and arsenic methylation in rhizosphere controls arsenic fate in soil-rice system after straw incorporation. Environ Pollut 236:598–608. https://doi.org/10.1016/j.envpol.2018.01.099
Yao B-M, Wang S-Q, Xie S-T, et al (2021) Optimal soil Eh, pH for simultaneous decrease of bioavailable Cd, As in co-contaminated paddy soil under water management strategies. Sci Total Environ 151342. https://doi.org/10.1016/j.scitotenv.2021.151342
Zakharyan RA, Aposhian HV (1999) Arsenite methylation by methylvitamin B12 and glutathione does not require an enzyme. Toxicol Appl Pharmacol 154:287–291. https://doi.org/10.1006/taap.1998.8587
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794. https://doi.org/10.1111/j.1469-8137.2008.02716.x
Zhao FJ, Wang P (2020) Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 446:1–21. https://doi.org/10.1007/s11104-019-04374-6
Zhao FJ, Zhu YG, Meharg AA (2013) Methylated arsenic species in rice: Geographical variation, origin, and uptake mechanisms. Environ Sci Technol 47:3957–3966. https://doi.org/10.1021/es304295n
Zheng MZ, Li G, Sun GX et al (2013) Differential toxicity and accumulation of inorganic and methylated arsenic in rice. Plant Soil 365:227–238. https://doi.org/10.1007/s11104-012-1376-3
Acknowledgements
We thank Bruce Ravel for assistance with Fe EXAFS analysis and Chin Chen Kuo for assistance with ICP-MS analysis. This work was partially supported by NIFA (Grant No. 2018-67013-27455 and 2018-67019-22796), the NSF (Grant No. 1930806), and a joint seed grant to Seyfferth and Tappero to support collaboration between the University of Delaware and Brookhaven National Laboratory. Parts of this research used the XFM (4-BM) Beamline and BMM (6-BM) Beamline of the National Synchrotron Radiation Lightsource II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich. This work was partially supported by NIFA (Grant No. 2018–67013-27455), NSF (Grant No. 1930806), and a joint seed grant to Seyfferth and Tappero to support collaboration between the University of Delaware and Brookhaven National Laboratory.
Author information
Authors and Affiliations
Contributions
F. Linam conducted pot study, data analysis, and manuscript preparation. M. Limmer provided project ideation, training, data analysis, and manuscript preparation. R. Tappero conducted synchrotron imaging of rice grains. A. Seyfferth provided project ideation, progress analysis, technical guidance, and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Martin J. Hodson.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Linam, F., Limmer, M.A., Tappero, R. et al. Rice husk and charred husk amendments increase porewater and plant Si but water management determines grain As and Cd concentration. Plant Soil 477, 135–152 (2022). https://doi.org/10.1007/s11104-022-05350-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05350-3