Abstract
Objectives
To assess changes in the health status of men with lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH) using the EQ-5D-3L and OAB-5D instruments and to evaluate the sensitivity of the instruments.
Methods
Data were available from a large randomised phase III trial of men with moderate-to-severe storage and voiding LUTS/BPH (NEPTUNE). Men received a fixed-dose combination of solifenacin 6 mg plus oral controlled absorption system (OCAS™) formulation of tamsulosin (TOCAS, 0.4 mg), TOCAS monotherapy or placebo and completed the EQ-5D-3L and OAB-5D at baseline and weeks 4, 8 and 12. Analysis of covariance was used to estimate changes in EQ-5D-3L Index, EQ-VAS and OAB-5D. Changes in dimension level were summarised using the Paretian Classification of Health Change (PCHC).
Results
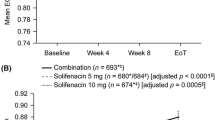
Improved health-related quality of life from baseline was seen in all treatment arms on EQ-5D-3L and OAB-5D at week 12, although only OAB-5D showed statistically significant differences between active treatment and placebo, both on the index score and using the PCHC approach. Effect sizes in the active treatment groups were large (>0.8) on OAB-5D but small (≈0.2) on EQ-5D-3L. EQ-5D-3L showed a very high ceiling effect (45% of men reported full health at baseline) and a substantial proportion of these men reported improvements at week 12 in several dimensions of OAB-5D.
Conclusions
A large ceiling effect on EQ-5D-3L substantially limited its sensitivity in this population. OAB-5D proved more sensitive to changes in health status and could be considered a complement to ED-5D-3L as a source of utilities for health economic modelling.




Similar content being viewed by others
References
Abrams, P., Manson, J., & Kirby, M. G. (2012). Incidence and epidemiology of storage lower urinary tract symptoms. European Urological Review, 7, 50–54.
Peters, T. J., Donovan, J. L., Kay, H. E., Abrams, P., de la Rosette, J. J., Porru, D., et al. (1997). The International Continence Society “benign prostatic hyperplasia” study: The bothersomeness of urinary symptoms. Journal of Urology, 157, 885–889.
Gravas, S., Bachmann, A., Descazeaud, A., Drake, M., Gratzke, C., Madersbacher, S., et al. (2014). Guidelines on the management of non-neurogenic male lower urinary tract symptoms (LUTS), incl. Benign prostatic obstruction (BPO). Available at: http://uroweb.org/wp-content/uploads/EAU-Guidelines-Non-Neurogenic-Male-LUTS-Guidelines-2015-v2.pdf. Last Accessed 20 October 2015.
NICE CG97 Lower urinary tract symptoms: Full guideline (2010). Available at: https://www.nice.org.uk/guidance/cg97/evidence/full-guideline-245363870. Last Accessed 20 October 2015.
Garraway, W. M., Russell, E. B., Lee, R. J., Collins, G. N., McKelvie, G. B., Hehir, M., et al. (1993). Impact of previously unrecognized benign prostatic hyperplasia on the daily activities of middle-aged and elderly men. British Journal of General Practice, 43, 318–321.
McVary, K. T. (2006). BPH: Epidemiology and comorbidities. American Journal of Managed Care, 12, S122–S128.
Schipper, H., Clinch, J. J., & Olweny, C. L. M. (1996). Quality of life studies: Definitions and conceptual issues. In B. Spilker (Ed.), Quality of life and pharmacoeconomics in clinical trials. Philadelphia: Lippincott-Raven Publishers.
Welch, G., Weinger, K., & Barry, M. J. (2002). Quality-of-life impact of lower urinary tract symptom severity: Results from the health professionals follow-up study. Urology, 59, 245–250.
Coyne, K. S., Wein, A. J., Tubaro, A., Sexton, C. C., Thompson, C. L., Kopp, Z. S., et al. (2009). The burden of lower urinary tract symptoms: Evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU International, 103, 4–11.
Wein, A. J., Coyne, K. S., Tubaro, A., Sexton, C. C., Kopp, Z. S., & Aiyer, L. P. (2009). The impact of lower urinary tract symptoms on male sexual health: EpiLUTS. BJU International, 103, 33–41.
Trueman, P., Hood, S. C., Nayak, U. S., & Mrazek, M. F. (1999). Prevalence of lower urinary tract symptoms and self-reported diagnosed ‘benign prostatic hyperplasia’, and their effect on quality of life in a community-based survey of men in the UK. BJU International, 83, 410–415.
Girman, C. J., Jacobsen, S. J., Tsukamoto, T., Richard, F., Garraway, W. M., Sagnier, P. P., et al. (1998). Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology, 51, 428–436.
Boyle, P., Robertson, C., Mazzetta, C., Keech, M., Hobbs, R., Fourcade, R., et al. (2003). The relationship between lower urinary tract symptoms and health status: The UREPIK study. BJU International, 92, 575–580.
Lukacs, B., Cornu, J. N., Aout, M., Tessier, N., Hodée, C., Haab, F., et al. (2013). Management of lower urinary tract symptoms related to benign prostatic hyperplasia in real-life practice in France: A comprehensive population study. European Urology, 64, 493–501.
Drummond, M. F., Sculpher, M. J., Torrance, G. W., O’Brien, B. J., & Stoddart, G. L. (2005). Methods for the economic evaluation of health care programmes (3rd ed.). Oxford: Oxford University Press.
EQ-5D-3L User Guide. Version 5.1, April 2015. Available at http://www.euroqol.org/fileadmin/user_upload/Documenten/PDF/Folders_Flyers/EQ-5D-3L_UserGuide_2015.pdf. Last Accessed 20 October 2015.
Castro-Díaz, D., Callejo, D., Cortés, X., & Pérez, M. (2014). Study of quality of life in patients with benign prostatic hyperplasia under treatment with silodosin. Actas Urológicas Españolas, 38, 361–366.
Fourcade, R. O., Lacoin, F., Rouprêt, M., Slama, A., Le Fur, C., Michel, E., et al. (2012). Outcomes and general health-related quality of life among patients medically treated in general daily practice for lower urinary tract symptoms due to benign prostatic hyperplasia. World Journal of Urology, 30, 419–426.
Coyne, K., Revicki, D., Hunt, T., Corey, R., Stewart, W., Bentkover, J., et al. (2002). Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: The OAB-q. Quality of Life Research, 11, 563–574.
Young, T., Yang, Y., Brazier, J. E., Tsuchiya, A., & Coyne, K. (2009). The first stage of developing preference-based measures: Constructing a health-state classification using Rasch analysis. Quality of Life Research, 18, 253–265.
Desroziers, K., Aballéa, S., Maman, K., Nazir, J., Odeyemi, I., & Hakimi, Z. (2013). Estimating EQ-5D and OAB-5D health state utilities for patients with overactive bladder. Health and Quality of Life Outcomes, 11, 200.
van Kerrebroeck, P., Chapple, C., Drogendijk, T., Klaver, M., Sokol, R., Speakman, M., et al. (2013). Combination therapy with solifenacin and tamsulosin oral controlled absorption system in a single tablet for lower urinary tract symptoms in men: Efficacy and safety results from the randomised controlled NEPTUNE trial. European Urology, 64, 1003–1012.
Electronic Medicines Compendium. Vesomni 6 mg/0.4 mg modified release tablets: Summary of product characteristics. Available at: https://www.medicines.org.uk/emc/medicine/28535. Last Accessed 20 October 2015.
Chapple, C. R., Drake, M. J., van Kerrebroeck, P., Cardozo, L., Drogendijk, T., Klaver, M., et al. (2014). Total urgency and frequency score as a measure of urgency and frequency in overactive bladder and storage lower urinary tract symptoms. BJU International, 113, 696–703.
Drake, M. J., Sokol, R., Coyne, K., Hakimi, Z., Nazir, J., Dorey, J., et al. (2016). Responder and health-related quality of life analyses in men with lower urinary tract symptoms treated with a fixed-dose combination of solifenacin and tamsulosin oral-controlled absorption system: Results from the NEPTUNE study. BJU International, 117, 165–172.
Matza, L. S., Thompson, C. L., Krasnow, J., Brewster-Jordan, J., Zyczynski, T., & Coyne, K. S. (2005). Test-retest reliability of four questionnaires for patients with overactive bladder: The overactive bladder questionnaire (OAB-q), patient perception of bladder condition (PPBC), urgency questionnaire (UQ), and the primary OAB symptom questionnaire (POSQ). Neurourology and Urodynamics, 24, 215–225.
Coyne, K. S., Matza, L. S., & Thompson, C. L. (2005). The responsiveness of the Overactive Bladder Questionnaire (OAB-q). Quality of Life Research, 14, 849–855.
Yang, Y., Brazier, J., Tsuchiya, A., & Coyne, K. (2009). Estimating a preference-based single index from the Overactive Bladder Questionnaire. Value in Health, 12, 159–166.
Gudex, C. (2005). The descriptive system of the EuroQol instrument. In P. Kind, R. Brooks, & R. Rabin (Eds.), EQ-5D concepts and methods: A developmental history. Berlin: Springer.
Dolan, P. (1997). Modeling valuations for EuroQol health states. Medical Care, 35, 1095–1108.
Walters, S. J., & Brazier, J. E. (2005). Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Quality of Life Research, 14, 1523–1532.
Hays, R. D., & Revicki, D. (2005). Reliability and validity (including responsiveness). In P. Fayers & R. Hays (Eds.), Assessing quality of life in clinical trials: Methods and practice (2nd ed.). New York: Oxford University Press.
Cohen, J. (1998). Statistical power analysis for the behavioural sciences (2nd ed.). Hillsdale, NJ: L. Erlbaum Associates.
Devlin, N. J., Parkin, D., & Browne, J. (2010). Patient-reported outcome measures in the NHS: New methods for analysing and reporting EQ-5D data. Health Economics, 19, 886–905.
Parkin, D., Devlin, N., & Rice, N. (2010). Statistical analysis of EQ-5D profiles: Does the use of value sets bias inference? Medical Decision Making, 30, 556–565.
Pavesi, M., Devlin, N., Hakimi, Z., Nazir, J., Herdman, M., Hoyle, C., et al. (2013). Understanding the effects on HR-QoL of treatment for overactive bladder: A detailed analysis of EQ-5D clinical trial data for mirabegron. Journal of Medical Economics, 16, 866–876.
Buckley, B.S., Lapitan, M.C., Glazener, C.M.; MAPS Trial Group. (2012). The effect of urinary incontinence on health utility and health-related quality of life in men following prostate surgery. Neurourology and Urodynamics, 31, 465–469.
Patrick, D. L., & Deyo, R. A. (1989). Generic and disease-specific measures in assessing health status and quality of life. Medical Care, 27, S217–S232.
Wiebe, S., Guyatt, G., Weaver, B., Matijevic, S., & Sidwell, C. (2003). Comparative responsiveness of generic and specific quality-of-life instruments. Journal of Clinical Epidemiology, 56, 52–60.
NICE PMG9 Guide to the methods of technology appraisal (2013). Available at: http://publications.nice.org.uk/pmg9. Last Accessed 20 October 2015.
Acknowledgements
Medical writing support was provided by Tyrone Daniel of Bioscript Medical and was funded by Astellas Pharma Europe Ltd.
Funding
Astellas funded the research, but the publication of study results was not contingent on the sponsor’s approval or censorship of the manuscript.
Author contributions
ZH, IO and CH contributed to the concept and design of the study; all authors contributed to the analysis and interpretation of the data; all authors provided direction for the content, critically revised the publication for intellectual content and approved the final version for submission. This research was funded by Astellas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ZH, JH and IO are employees of Astellas Pharma. MH and ND are employees of Office of Health Economics, which was contracted by Astellas Pharma to support the conduct of this study. CH was employed by Office of Health Economics at the time of the analysis and is now employed by AstraZeneca.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hakimi, Z., Herdman, M., Pavesi, M. et al. Using EQ-5D-3L and OAB-5D to assess changes in the health-related quality of life of men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Qual Life Res 26, 1187–1195 (2017). https://doi.org/10.1007/s11136-016-1460-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-016-1460-x