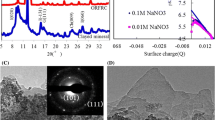
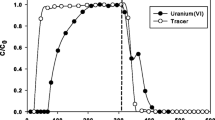
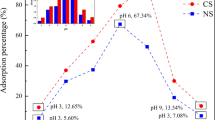
Abstract
Uranium (U) is a common contaminant at numerous surface and subsurface sites in proximity to areas involved with weapons manufacturing and atomic energy related activities. This paper covers some important aspects of the aqueous hexavalent uranium [U(VI)] interactions with soil minerals that are present in contaminated soils and sediments. The retention of U via interactions with soil minerals has significant consequences for the prediction of its short- and long-term behavior in soils and geological systems. Studies of the nature and type of these interactions have provided the necessary evidence for assessing the geochemical behavior of U in natural systems under different physical, biogeochemical, hydrological, and reducing or oxidizing conditions. Over the last 20 years, aqueous U(VI): soil mineral interactions have been studied by geochemists, soil chemists, clay and soil mineralogists, and the progress in some areas is remarkable. Although a mechanistic description and understanding of the complex interactions involving U and soil minerals in natural systems is currently difficult, results from carefully designed and executed field and laboratory experiments with these materials have improved our understanding of the heterogeneous system’s behavior and U contaminant mobility and transport. There are, however, areas that warrant further exploration and study. Numerous research publications were reviewed in this paper to present recent important findings to reveal the current level of the understanding of the U(VI) interactions with soil minerals, and to provide ideas for future needs and research directions.
Similar content being viewed by others
References
Allison JD, Brown DS, Novo-Gradac KJ (1991) MINTEQA2/PRODEFA2, A geochemical assessment model for environmental systems: version 3.0 user’s manual. Environmental Protection Agency, Athens
Allison JD, Brown DS, Novo-Gradac KJ (1998) MINTEQA2/PRODEFA2, A geochemical assessment model for environmental systems: user manual supplement for version 4.0. Environmental Protection Agency, Athens
Ames LL, McGarrah JE, Walker BA (1983) Sorption of uranium and radium by biotite, muscovite, and phlogopite. Clays Clay Miner 31:343–351. doi:10.1346/CCMN.1983.0310503
Anderson LD, Kent DB, Davis JA (1994) Batch experiments characterizing the reduction of Cr(VI) using suboxic material from a mildly reducing sand and gravel aquifer. Environ Sci Technol 28:178–185. doi:10.1021/es00050a025
Anderson RT, Vrionis HA, Ortiz-Bernad I, Resch CT, Long PE, Dayvault R et al (2003) Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl Environ Microbiol 69:5884–5891. doi:10.1128/AEM.69.10.5884-5891.2003
Arai Y, McBeath M, Bargar JR, Joye J, Davis JA (2006) Uranyl adsorption and surface speciation at the imogolite-water interface: self-consistent spectroscopic and surface complexation models. Geochim Cosmochim Acta 70:2492–2509. doi:10.1016/j.gca.2006.02.013
Arai Y, Marcus MK, Tamura N, Davis JA, Zachara JM (2007) Spectroscopic evidence for uranium bearing precipitates in vadose zone sediments at the Hanford 300-area site. Environ Sci Technol 41:4633–4639. doi:10.1021/es062196u
Arnold T, Zorn T, Benhard G, Nitsche H (1998) Sorption of uranium(VI) onto phyllite. Chem Geol 151:129–141. doi:10.1016/S0009-2541(98)00075-8
Arnold T, Zorn T, Zanker H, Bernhard G, Nitsche H (2001) Sorption behavior of U(VI) on phyllite: experiments and modeling. J Contam Hydrol 47:219–231. doi:10.1016/S0169-7722(00)00151-0
Arnold T, Utsunomiya S, Geipel G, Ewing RC, Baumann N, Brendler V (2006) Adsorbed U(VI) surface species on muscovite identified by laser fluorescence spectroscopy and transmission electron microscopy. Environ Sci Technol 40:4646–4652. doi:10.1021/es052507l
Baik MH, Cho WJ, Han PS (2004a) Sorption of U(VI) onto granite surfaces: a kinetic approach. J Radioanal Nucl Chem 260:495–502. doi:10.1023/B:JRNC.0000028207.55356.ec
Baik MH, Hyun SP, Cho WJ, Hahn PS (2004b) Contribution of minerals to the sorption of U(VI) on granite. Radiochim Acta 92:663–669. doi:10.1524/ract.92.9.663.54980
Bargar JR, Reitmeyer R, Davis JA (1999) Spectroscopic confirmation of uranium(VI)-carbonato adsorption complexes on hematite. Environ Sci Technol 33:2481–2484. doi:10.1021/es990048g
Bargar JR, Reitmeyer R, Lenhart JJ, Davis JA (2000) Characterization of U(VI)-carbonate ternary complexes on hematite: EXAFS and electrophoretic mobility measurements. Geochim Cosmochim Acta 64:2737–2749. doi:10.1016/S0016-7037(00)00398-7
Baumann N, Brendler V, Arnold T, Geipel G, Bernhard G (2005) Uranyl sorption onto gibbsite studied by time-resolved laser-induced fluorescence spectroscopy (TRUS). J Coll Inter Sci 290:318–324
Benes P, Kratzer K, Vlckova S, Sebestova E (1998) Adsorption of uranium on clay and the effect of humic substances. Radiochim Acta 82:367–373
Bernhard G, Geipel G, Reich T, Brendler V, Amayri S, Nitsche H (2001) Uranyl(VI) carbonate complex formation: validation of the Ca2UO2(CO3)3 (aq.) species. Radiochim Acta 89:511–518. doi:10.1524/ract.2001.89.8.511
Bigham JM, Fitzpatrick RW, Schulze DG (2002) Iron oxides. In: Dixon JB, Schulze DG (eds) Soil mineralogy with environmental applications, vol SSSA Book Series No. 7. Soil Science Society of America, Inc., Madison, WI, pp 323–366
Bond DL, Davis JA, Zachara JM (2007) Uranium(VI) release from contaminated vadose zone sediments: estimation of potential contributions from dissolution and desorption. In: Barnett MO, Kent DB (eds) Adsorption of metals by Geomedia II. Academic Press, San Diego, pp 379–420
Bostick BC, Fendorf S, Barnett MO, Jardine PM, Brooks SC (2002) Uranyl surface complexes formed on subsurface media from DOE facilities. Soil Sci Soc Am J 66:99–108
Boyanov MI, O’Loughlin EJ, Roden EE, Fein JB, Kemner KM (2007) Adsorption of Fe(II) and U(VI) to carboxyl-functionalized microspheres: the influence of speciation on uranyl reduction studied by titration and XAFS. Geochim Cosmochim Acta 71:1898–1912. doi:10.1016/j.gca.2007.01.025
Braithwaite A, Livens FR, Richardson S, Howe MT, Goulding KWT (1997) Kinetically controlled release of uranium from soils. Eur J Soil Sci 48:661–673. doi:10.1046/j.1365-2389.1997.00121.x
Braithwaite A, Richardson S, Moyes LN, Livens FR, Bunker DJ, Hughes CR (2000) Sorption kinetics of uranium-238 and neptunium-237 on glacial sediment. Czech J Phys 50:265–269. doi:10.1023/A:1022842808820
Brooks SC, Fredrickson JK, Carroll SL, Kennedy DW, Zachara JM, Plymale AE et al (2003) Inhibition of bacterial U(VI) reduction by calcium. Environ Sci Technol 37:1850–1858. doi:10.1021/es0210042
Carroll SA, Bruno J (1991) Mineral-solution interactions in the U(VI)-CO2-H2O system. Radiochim Acta 52(53):187–193
Carroll SA, Bruno J, Petit J-C, Dran J-C (1992) Interactions of U(VI), Nd, and Th(IV) at the calcite-solution interface. Radiochim Acta 58(59):245–252
Catalano JG, Brown GE (2004) Analysis of uranyl-bearing phases by EXAFS spectroscopy: interferences, multiple scattering, accuracy of structural parameters, and spectral differences. Am Min 89:1004–1021
Catalano JG, Brown GE (2005) Uranyl adsorption onto montmorillonite: evaluation of binding sites and carbonate complexation. Geochim Cosmochim Acta 69:2995–3005. doi:10.1016/j.gca.2005.01.025
Catalano JG, McKinley JP, Zachara JM, Heald SM, Smith SC, Brown GE (2006) Changes in uranium speciation through a depth sequence of contaminated Hanford sediments. Environ Sci Technol 40:2517–2524. doi:10.1021/es0520969
Chadwick O, Nettleton WD (1990) Micromorphologic evidence of adhesive and cohesive forces in soil cementation. Soil Sci 19:207–212. doi:10.1016/S0166-2481(08)70332-5
Charlet L, Silvester E, Liger E (1998) N-compound reduction and actinide immobilization in surfacial fluids by Fe(II):the surface =FeIIOFeIIOH0 species, as major reductant. Chem Geol 151:85–93. doi:10.1016/S0009-2541(98)00072-2
Cheng T, Barnett MO, Roden EE, Zhuang JL (2006) Effects of solid-to-solution ratio on uranium(VI) adsorption and its implications. Environ Sci Technol 40:3243–3247. doi:10.1021/es051771b
Clark DL, Hobart DE, Neu MP (1995) Actinide carbonate complexes and their importance in actinide environmental chemistry. Chem Rev 95:25–48. doi:10.1021/cr00033a002
Cornell RM, Schwertmann U (1996) The iron oxides. VCH Publ, Weinheim
Cornell RM, Schwertmann U (2003) The iron oxides. Structure, properties, reactions, occurrences and uses, 2nd edn. WILEY-VCH
Culver TB, Hallisey SP, Sahoo D, Deitsch JJ, Smith JA (1997) Modeling the desorption of organic contaminants from long-term contaminated soil using distributed mass transfer rates. Environ Sci Technol 31:1581–1588. doi:10.1021/es9600946
Curtis GP, Fox P, Kohler M, Davis JA (2004) Comparison of in situ uranium Kd values with a laboratory determined surface complexation model. Appl Geochem 19:1643–1653. doi:10.1016/j.apgeochem.2004.03.004
Curtis GP, Davis JA, Naftz DL (2006) Simulation of reactive transport of uranium(VI) in groundwater with variable chemical conditions. Water Resour Res 42:W04404. doi:10.1029/2005WR003979
Davis JA (2001) Surface complexation modeling of uranium(VI) adsorption on natural mineral assemblages. U.S. Geological Survey, NUREG/CR–6708
Davis JA, Curtis GP (2003) Application of surface complexation modeling to describe uranium(VI) adsorption and retardation at the uranium mill tailings site at Naturita, Colorado. NUREG Report CR-6820. U.S. Nuclear Regulatory Commission, Rockville
Davis JA, Meece DE, Kohler M, Curtis GP (2004) Approaches to surface complexation modeling of Uranium(VI) adsorption on aquifer sediments. Geochim Cosmochim Acta 68:3621–3641. doi:10.1016/j.gca.2004.03.003
Davis JA, Curtis GP, Wilkins MJ, Kohler M, Fox P, Naftz DL et al (2006) Processes affecting transport of uranium in a suboxic aquifer. Phys Chem Earth 31:548–555
de Jong WA, Apra E, Windus TL, Nichols JA, Harrison RJ, Gutowski KE et al (2005) Complexation of the carbonate, nitrate, and acetate anions with the uranyl dication: density functional studies with relativistic effective core potentials. J Phys Chem A 109:11568–11577. doi:10.1021/jp0541462
Dodge CJ, Francis AJ, Gillow JB, Halada GP, Eng C, Clayton CR (2002) Association of uranium with iron oxides typically formed on corroding steel surfaces. Environ Sci Technol 36:3504–3511. doi:10.1021/es011450+
Dong WM, Brooks SC (2006) Determination of the formation constants of ternary complexes of uranyl and carbonate with alkaline earth metals (Mg2+, Ca2+, Sr2+, and Ba2+) using anion exchange method. Environ Sci Technol 40:4689–4695. doi:10.1021/es0606327
Dong W, Ball WP, Liu C, Wang Z, Stone AT, Bai J et al (2005) Influence of calcite and dissolved calcium on uranium(VI) sorption to a Hanford subsurface sediment. Environ Sci Technol 39:7949–7955. doi:10.1021/es0505088
Dong WM, Xie GB, Miller TR, Franklin MP, Oxenberg TP, Bouwer EJ et al (2006) Sorption and bioreduction of hexavalent uranium at a military facility by the Chesapeake Bay. Environ Pollut 142:132–142. doi:10.1016/j.envpol.2005.09.008
Duff MC, Amrhein C (1996) Uranium(VI) adsorption on goethite and soil in carbonate solutions. Soil Sci Soc Am J 60:1393–1400
Duff MC, Hunter DB, Bertsch PM, Amrhein C (1999) Factors influencing uranium reduction and solubility in evaporation pond sediments. Biogeochem 45:95–114
Duff MC, Coughlin JU, Hunter DB (2002) Uranium co-precipitation with iron oxide minerals. Geochim Cosmochim Acta 66:3533–3547. doi:10.1016/S0016-7037(02)00953-5
Elsner M, Schwarzenbach RP, Haderlein SB (2004a) Reactivity of Fe(II)-bearing minerals toward reductive transformation of organic contaminants. Environ Sci Technol 38:799–807. doi:10.1021/es0345569
Elsner M, Haderlein SB, Kellerhais T et al (2004b) Mechanisms and products of surface-mediated reductive dehalogenation of carbon tetrachloride by Fe(II) on goethite. Environ Sci Technol 38:2058–2066. doi:10.1021/es034741m
Elzinga EJ, Tait CD, Reeder RJ, Rector KD, Donohoe RJ, Morris DE (2004) Spectroscopic investigation of U(VI) sorption at the calcite-water interface. Geochim Cosmochim Acta 68:2437–2448. doi:10.1016/j.gca.2003.09.023
Finch RJ, Murakami T (1999) Systematics, Paragenesis of U Minerals. In: Burns PC, Finch RJ (eds) Uranium: mineralogy, geochemistry and the environment, vol 38. Mineralogical Society of America, Washington, pp 91–179
Fox PM, Davis JA, Zachara JM (2006) The effect of calcium on aqueous uranium(VI) speciation and adsorption to ferrihydrite and quartz. Geochim Cosmochim Acta 70:1379–1387. doi:10.1016/j.gca.2005.11.027
Fredrickson JK, Zachara JM, Kennedy DW, Duff MC, Gorby YA, Li SMW et al (2000) Reduction of U(VI) in goethite (alpha-FeOOH) suspensions by a dissimilatory metal-reducing bacterium. Geochim Cosmochim Acta 64:3085–3098. doi:10.1016/S0016-7037(00)00397-5
Fredrickson JK, Zachara JM, Kennedy DW, Kukkadapu R, McKinley JP, Heald SM et al (2004) Reduction of TcO4 − by sediment-associated biogenic Fe(II). Geochim Cosmochim Acta 68:3171–3187. doi:10.1016/j.gca.2003.10.024
Froideval A, Del Nero M, Barillon R, Hommet J, Mignot G (2003) pH dependence of uranyl retention in a quartz/solution system: an XPS study. J Coll Inter Sci 266:221–235. doi:10.1016/S0021-9797(03)00528-9
Fuller CC, Bargar JR, Davis JA, Piana MJ (2002) Mechanisms of uranium interactions with hydroxyapatite: implications for groundwater remediation. Environ Sci Technol 36:158–165. doi:10.1021/es0108483
Fuller CC, Bargar JR, Davis JA (2003) Molecular-scale characterization of uranium sorption by bone apatite materials for a permeable reactive barrier demonstration. Environ Sci Technol 37:4642–4649. doi:10.1021/es0343959
Gabriel U, Gaudet JP, Spadini L, Charlet L (1998) Reactive transport of uranyl in a goethite column: an experimental and modeling study. Chem Geol 151:107–128. doi:10.1016/S0009-2541(98)00074-6
Gamerdinger AP, Kaplan DI (2000) Application of a continuous-flow centrifugation method for solute transport in disturbed, unsaturated sediments and illustration of mobile-immobile water. Water Resour Res 36:1747–1755. doi:10.1029/2000WR900063
Gamerdinger AP, Kaplan DI, Wellman DM, Serne JN (2001a) Two-region flow and decreased sorption of uranium(VI) during transport in Hanford groundwater and unsaturated sands. Water Resour Res 37:3155–3162. doi:10.1029/2001WR000247
Gamerdinger AP, Kaplan DI, Wellman DM, Serne JN (2001b) Two-region flow and rate-limited sorption of uranium(VI) during transport in an unsaturated silt loam. Water Resour Res 37:3147–3153. doi:10.1029/2001WR000244
Geipel G, Reich T, Brendler V, Bernhard G, Nitsche H (1997) Laser and X-ray spectroscopic studies of uranium-calcite interface phenomena. J Nucl Mater 248:408–411. doi:10.1016/S0022-3115(97)00136-0
Giammar DE, Hering JG (2001) Time scales for sorption-desorption and surface precipitation of uranyl on goethite. Environ Sci Technol 35:3332–3337. doi:10.1021/es0019981
Giaquinta DM, Soderholm L, Yuchs SE, Wasserman SR (1997) The speciation of uranium in a smectite clay: evidence for catalysed uranyl reduction. Radiochim Acta 76:113–121
Gorby YA, Lovley DR (1992) Enzymatic uranium precipitation. Environ Sci Technol 26:205–207. doi:10.1021/es00025a026
Gorman-Lewis D, Elias PE, Fein JB (2005) Adsorption of aqueous uranyl complexes onto Bacillus subtilis cells. Environ Sci Technol 39:4906–4912. doi:10.1021/es047957c
Greathouse JA, Cygan RT (2005) Molecular dynamics simulation of uranyl(VI) adsorption equilibria onto an external montmorillonite surface. Phys Chem Chem Phys 7:3580–3586. doi:10.1039/b509307d
Greathouse JA, Cygan RT (2006) Water structure and aqueous uranyl(VI) adsorption equilibria onto external surfaces of beidellite, montmorillonite, and pyrophyllite: results from molecular simulations. Environ Sci Technol 40:3865–3871. doi:10.1021/es052522q
Greathouse JA, O’Brien RJ, Bemis G, Pabalan RT (2002) Molecular dynamics study of aqueous uranyl interactions with quartz(010). J Phys Chem B 106:1646–1655. doi:10.1021/jp013250q
Grenthe I, Fuger J, Konings RJM, Lemire RJ, Muller AB, Nguyen-Trung C et al (1992) Chemical thermodynamics of uranium. North Holland, Amsterdam
Haggerty R, Gorelick SM (1995) Multiple-rate mass transfer for modeling diffusion and surface reactions in media with pore-scale heterogeneity. Water Resour Res 31:2383–2400
Haggerty R, Gorelick SM (1998) Modeling mass transfer processes in soil column with pore-scale heterogeneity. Soil Sci Soc Am J 62:62–74
Haggerty R, Harvey CF, von Schwerin CF, Meigs LC (2004) What controls the apparent timescale of solute mass transfer in aquifers and soils? A comparison of experimental results. Water Resour Res 40:W01510. doi:10.1029/2002WR001716
Harvey CF, Gorelick SM (1995) Temporal moment-generating equations: modeling transport and mass transfer in heterogeneous aquifers. Water Resour Res 31:1895–1911. doi:10.1029/95WR01231
Harvey JW, Saiers JE, Newlin JT (2005) Solute transport and storage mechanisms in wetlands of the Everglades, south Florida. Water Resour Res 41(W05009):1–14. doi: 10.1029/2004WR003507
Hemond HF, Fechner-Levy EJ (2000) Chemical fate and transport in the environment. Academic Press, San Diego
Ho CH, Miller NH (1986) Adsorption of uranyl species from bicarbonate solution onto hematite particles. J Colloid Interface Sci 110:165–171. doi:10.1016/0021-9797(86)90365-6
Hollenbeck KJ, Harvey CF, Haggerty R, Werth CJ (1999) A method for estimating distribution of mass transfer rate coefficients with application to purging and batch experiments. J Contam Hydrol 37:367–388. doi:10.1016/S0169-7722(98)00165-X
Hsi CD, Langmuir D (1985) Adsorption of uranyl onto ferric oxyhydroxides: application of the surface complexation site-binding model. Geochim Cosmochim Acta 49:1931–1941. doi:10.1016/0016-7037(85)90088-2
Hyun SP, Cho YH, Hahn PS, Kim SJ (2001) Sorption mechanism of U(VI) on a reference montmorillonite: binding to the internal and external surfaces. J Radioanal Nucl Chem 250:55–62. doi:10.1023/A:1013212130177
Ilton ES, Haiduc A, Moses CO, Heald SM, Elbert DC, Veblen DR (2004) Heterogeneous reduction of uranyl by micas: crystal chemical and solution controls. Geochim Cosmochim Acta 68:2417–2435. doi:10.1016/j.gca.2003.08.010
Ilton ES, Qafoku NP, Liu CX, Moore DA, Zachara JM (2008) Advective removal of intraparticle uranium from contaminated Vadose zone sediments, Hanford, US. Environ Sci Technol 42:1565–1571. doi:10.1021/es071113m
Jang JH, Dempsey BA, Burgos WD (2007) A model-based evaluation of sorptive reactivities of hydrous ferric oxide and hematite for U(VI). Environ Sci Technol 41:4305–4310. doi:10.1021/es070068f
Jordan RB (1998) Reaction mechanisms of inorganic and organometallic systems, 2nd edn. Oxford University Press Oxford, New York
Kalmykov SN, Choppin GR (2000) Mixed Ca2+/UO2 2+/CO3 2− complex formation at different ionic strengths. Radiochim Acta 88:603–606. doi:10.1524/ract.2000.88.9-11.603
Kampf N, Scheinost AC, Schulze DG (2000) Oxide minerals. In: Sumner ME (ed) Handbook of soil science. CRC Press, Boca Raton
Kaplan DI, Gervais TL, Krupka KM (1998) Uranium(VI) sorption to sediments under high pH and ionic strength conditions. Radiochim Acta 80:201–211
Kelly SD, Newville M, Cheng L, Kemner KM, Sutton SR, Fenter P et al (2003) Uranyl incorporation in natural calcite. Environ Sci Technol 37:1284–1287. doi:10.1021/es025962f
Kelly SD, Kemner KM, Brooks SC (2007) X-ray absorption spectroscopy identifies calcium-uranyl-carbonate complexes at environmental concentrations. Geochim Cosmochim Acta 71:821–834. doi:10.1016/j.gca.2006.10.013
Kilislioglu A, Bilgin B (2002) Adsorption of uranium on halloysite. Radiochim Acta 90:155–160. doi:10.1524/ract.2002.90.3_2002.155
Klupinski TP, Chin YP, Traina SJ (2004) Abiotic degradation of pentachloronitrobenzene by Fe(II): reaction on goethite and iron oxide nanoparticles. Environ Sci Technol 38:4353–4360. doi:10.1021/es035434j
Kohler M, Curtis GP, Kent DB, Davis JA (1996) Experimental investigation and modeling of uranium(VI) transport under variable chemical conditions. Water Resour Res 32:3539–3551
Kohler M, Curtis GP, Meece DE, Davis JA (2004) Methods for estimating adsorbed uranium(VI) and distribution coefficients of contaminated sediments. Environ Sci Technol 38:240–247. doi:10.1021/es0341236
Kohut CK, Warren CJ (2002) Chlorites. In: Dixon JB, Schulze DG (eds) Soil minerals with environmental applications. Soil Science Society of America, Inc., Madison, pp 531–584
Kowal-Fouchard A, Drot R, Simoni E, Ehrhardt JJ (2004) Use of spectroscopic techniques for uranium(VI)/montmorillonite interaction modeling. Environ Sci Technol 38:1399–1407. doi:10.1021/es0348344
Krepelova A, Sachs S, Bernhard G (2006) Uranium(VI) sorption onto kaolinite in the presence and absence of humic acid. Radiochim Acta 94:825–833. doi:10.1524/ract.2006.94.12.825
Krepelova A, Brendler V, Sachs S, Baumann N, Bernhard G (2007) U(VI)-kaolinite surface complexation in absence and presence of humic acid studied by TRLFS. Environ Sci Technol 41:6142–6147. doi:10.1021/es070419q
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, Upper Saddle River
Larese-Casanova P, Scherrer MM (2007) Fe(II) sorption on hematite: new insights based on spectroscopic measurements. Environ Sci Technol 41:471–477. doi:10.1021/es0617035
Lefevre G, Noinville S, Fedoroff M (2006) Study of uranyl sorption onto hematite by in situ attenuated total reflection-infrared spectroscopy. J Coll Inter Sci 296:608–613. doi:10.1016/j.jcis.2005.09.016
Li Z, Brusseau ML (2000) Nonideal transport of reactive solutes in heterogeneous porous media. 6. Microscopic and macroscopic approaches for incorporating heterogeneous rate-limited mass transfer. Water Resour Res 36:2853–2867. doi:10.1029/2000WR900089
Li Z, Barry DA, Culligan-Hensley PJ, Bajracharya K (1994) Mass transfer in soils with local stratification of hydraulic conductivity. Water Resour Res 30:2891–2900. doi:10.1029/94WR01218
Lichtner PC (1993) Scaling properties of time-space kinetic mass-transport equations and the local equilibrium limit. Am J Sci 293:257–296
Lichtner PC (1996) Continuum formulation of multicomponent-multiphase reactive transport. Reactive transport in porous media, vol 34. pp 1–81
Lichtner PC, Carey JW (2006) Incorporating solid solutions in reactive transport equations using a kinetic discrete-composition approach. Geochim Cosmochim Acta 70:1356–1378. doi:10.1016/j.gca.2005.11.028
Liger E, Charlet L, Van Cappellen P (1999) Surface catalysis of uranium(VI) reduction by iron(II). Geochim Cosmochim Acta 63:2939–2955. doi:10.1016/S0016-7037(99)00265-3
Lindberg JW, Peterson RE (2004) 300-FF-5 operable unit, Chapter 1.12 PNNL-14548. Pacific Northwest National Laboratory, Richland
Liu CX (2007) An ion diffusion model in semi-permeable clay materials. Environ Sci Technol 41:5403–5409. doi:10.1021/es0624117
Liu C, Zachara JM, Qafoku OS, McKinley JP, Heald SM, Wang Z (2004a) Dissolution of uranyl microprecipitates in subsurface sediments at Hanford Site, WA. Geochim Cosmochim Acta 68:4519–4537. doi:10.1016/j.gca.2004.04.017
Liu CX, Zachara JM, Felmy A, Gorby Y (2004b) An electrodynamics-based model for ion diffusion in microbial polysaccharides. Colloids Surf B Biointerfaces 38:55–65. doi:10.1016/j.colsurfb.2004.08.003
Liu C, Zachara JM, Yantasee W, Majors PD, McKinley JP (2006) Microscopic reactive diffusion of uranium in the contaminated sediments at the Hanford site, USA: characterization and modeling. Water Resour Res 42(12):W12420. doi:10.1029/2006WR005031
Livens FR, Jones MJ, Hynes AJ, Charnock JM, Mosselmans JFW, Hennig C et al (2004) X-ray absorption spectroscopy studies of reactions of technetium, uranium, and neptunium with mackinawite. J Environ Radioact 74:211–219. doi:10.1016/j.jenvrad.2004.01.012
Lovley DR, Phillips EJP (1992) Bioremediation of uranium contamination with enzymatic uranium reduction. Environ Sci Technol 26:2228–2234. doi:10.1021/es00035a023
Mason CFV, Turney WRJR, Thomson BM, Lu N, Longmire PA, Chisholm-Brause CJ (1997) Carbonate leaching of uranium from contaminated soils. Environ Sci Technol 31:2707–2711. doi:10.1021/es960843j
McKinley JP, Zachara JM, Liu CX, Heald SC, Prenitzer BI, Kempshall BW (2006) Microscale controls on the fate of contaminant uranium in the vadose zone, Hanford Site, Washington. Geochim Cosmochim Acta 70:1873–1887. doi:10.1016/j.gca.2005.10.037
Meece DE, Benninger LK (1993) The coprecipitation of Pu and other radionuclides with CaCO3. Geochim Cosmochim Acta 57:1447–1458. doi:10.1016/0016-7037(93)90005-H
Missana T, Maffiotte C, Garcia-Gutierrez M (2003) Surface reactions kinetics between nanocrystalline magnetite and uranyl. J Coll Inter Sci 261:154–160. doi:10.1016/S0021-9797(02)00227-8
Monger HC, Kelly EE (2002) Silica minerals. In: Dixon JB, Schulze DG (eds) Soil mineralogy with environmental applications. Soil Science Society of America, Inc., Madison, pp 611–636
Moon HS, Komlos J, Jaffe PR (2007) Uranium reoxidation in previously bioreduced sediment by dissolved oxygen and nitrate. Environ Sci Technol 41:4587–4592. doi:10.1021/es063063b
Morel FMM, Hering JG (1993) Principles and applications of aquatic chemistry. Wiley, New York
Morris DE (2002) Redox energetics and kinetics of uranyl coordination complexes in aqueous solution. Inorg Chem 41:3542–3547. doi:10.1021/ic0201708
Morse JW, Shanbbag PM, Saito A, Choppin GR (1984) Interaction of uranyl ions in carbonate media. Chem Geol 42:85–99. doi:10.1016/0009-2541(84)90007-X
Moyes LN, Parkman RH, Charnock JM, Vaughan DJ, Livens FR, Hughes CR et al (2000) Uranium uptake from aqueous solution by interaction with goethite, lepidocrocite, muscovite, and maskinawite: an X-ray absorption spectroscopy study. Environ Sci Technol 34:1062–1068. doi:10.1021/es990703k
Muckett C, Sherman D, Rognarsdottir V (1998) The adsorption of uranium onto goethite and clinochlore, pp 118–119. The Synchrotron Radiation Source Scientific Reports, Geology and Mineralogy: http://srs.dl.ac.uk/Annual_Reports/AnRep97_98/Annexe/118_Muskett.pdf
Murakami T, Sato T, Ohnuki T, Isobe H (2005) Field evidence for uranium nanocrystallization and its implications for uranium transport. Chem Geol 221:117. doi:10.1016/j.chemgeo.2005.04.004
Neal AL, Amonette JE, Peyton BM, Geesey GG (2004) Uranium complexes formed at hematite surfaces colonized by sulfate-reducing bacteria. Environ Sci Technol 38:3019–3027. doi:10.1021/es030648m
Newton TW (1975) The kinetics of the oxidation-reduction reactions of uranium, neptunium, plutonium, and americium in aqueous solutions. ERDA Critical Review Series (TID-26506), NTIS, Springfield, Virginia, ERDA Critical Review Series (TID-26506), NTIS, Springfield, Virginia
Noubactep C (2005) Effect of selected ligands on the U(VI) immobilization by zerovalent iron. J Radioanal Nucl Chem 267:13–19. doi:10.1007/s10967-006-0003-2
Noubactep C, Meinrath G, Dietrich P, Merkel B (2003) Mitigating uranium in groundwater: prospects and limitations. Environ Sci Technol 37:4304–4308. doi:10.1021/es034296v
Noubactep C, Meinrath G, Merkel BJ (2005) Investigating the mechanism of uranium removal by zerovalent iron. Environ Chem 2:235–242. doi:10.1071/EN05003
Noubactep C, Sonnefeld J, Sauter M (2006) Uranium release from a natural rock under near-natural oxidizing conditions. J Radioanal Nucl Chem 267:591–602. doi:10.1007/s10967-006-0092-y
O’Loughlin EJ, Kelly SD, Cook RE, Csencsits R, Kemner KM (2003) Reduction of uranium(VI) by mixed iron(II)/iron(III) hydroxide (green rust): formation of UO2 nanoparticles. Environ Sci Technol 37:721–727. doi:10.1021/es0208409
Pabalan RT, Turner DR (1997) Uranium(+6) sorption on montmorillonite: experimental and surface complexation modeling study. Aquat Geochem 2:203–226. doi:10.1007/BF00119855
Pabalan RT, Turner DR, Bertetti FP, Prikryl JD (1998) Uranium(VI) sorption onto selected mineral surfaces. In: Jenne EA (ed) Adsorption of metals by geomedia. Academic Press, San Diego, pp 99–130
Payne TE, Airey PL (2006) Radionuclide migration at the Koongarra uranium deposit, Northern Australia––Lessons from the Alligator Rivers analogue project. Phys Chem Earth 31:572–586
Payne TE, Lumpkin GR, Waite TD (1998) Uranium(VI) adsorption on model minerals. In: Jenne EA (ed) Adsorption of metals by geomedia. Academic Press, San Diego, pp 75–99
Payne TE, Davis JA, Lumpkin GR, Chisari R, Waite TD (2004) Surface complexation model of uranyl sorption on Georgia kaolinite. Appl Clay Sci 26:151–162. doi:10.1016/j.clay.2003.08.013
Pecher K, Haderlein SB, Schwarzenbach RP (2002) Reduction of polyhalogenated methanes by surface-bound Fe(II) in aqueous suspensions of iron oxides. Environ Sci Technol 36:1734–1741. doi:10.1021/es011191o
Phillippi JM, Loganathan VA, McIndoe MJ, Barnett MO, Clement TP, Roden EE (2007) Theoretical solid/solution ratio effects on adsorption and transport: uranium(VI) and carbonate. Soil Sci Soc Am J 71:329–335. doi:10.2136/sssaj2006.0159
Prikryl JD, Jain A, Turner DR, Pabalan RT (2001) Uranium(VI) sorption behavior on silicate mineral mixtures. J Contam Hydrol 47:241–253. doi:10.1016/S0169-7722(00)00153-4
Qafoku NP, Amonette JE (2003) Iron oxides. In: Lal R (ed) Encyclopedia of soil science, vol. Marcel Dekker, Inc., New York. doi:10.1081/E-ESS 120006595
Qafoku NP, Ainsworth CC, Szecsody JE, Bish DL, Young JS, McCready DE et al (2003) Aluminum effect on dissolution and precipitation under hyperalkaline conditions: II. Solid phase transformations. J Environ Qual 32:2364–2372
Qafoku NP, Zachara JM, Liu C, Gassman PL, Qafoku OS, Smith SC (2005) Kinetic desorption and sorption of U(VI) during reactive transport in a contaminated Hanford sediment. Environ Sci Technol 39:3157–3165. doi:10.1021/es048462q
Qafoku NP, Zachara JM, Liu C, Smith SC, Kukkadapu R, Gassman P et al (2008a) Factors controlling U(VI) adsorption and desorption rate and extent in two subsurface sediments of common provenance. Geochim Cosmochim Acta (to be submitted)
Qafoku NP, Zachara JM, Liu C, Wang Z, Arey B, Gassman P et al (2008b) Evaluation of U(VI) mobility in advancing and retreating zones of a deep vadose zone plume: the role of natural calcite. Geochim Cosmochim Acta (to be submitted)
Read D, Lawless TA, Sims RJ, Butter KR (1993) Uranium migration through intact sandstone cores. J Contam Hydrol 13:277–289. doi:10.1016/0169-7722(93)90066-2
Reeder RJ, Nugent M, Lamble GM, Tait CD, Morris DE (2000) Uranyl incorporation into calcite and aragonite: XAFS and luminescence studies. Environ Sci Technol 34:638–644. doi:10.1021/es990981j
Reeder RJ, Nugent M, Tait CD, Morris DE, Heald SM, Beck KM et al (2001) Coprecipitation of uranium(VI) with calcite: XAFS, micro-XAS, and luminescence characterization. Geochim Cosmochim Acta 65:3491–3503. doi:10.1016/S0016-7037(01)00647-0
Reeder RJ, Elzinga EJ, Tait CD, Rector KD, Donohoe RJ, Morris DE (2004) Site-specific incorporation of uranyl carbonate species at the calcite surface. Geochim Cosmochim Acta 68:4799–4808. doi:10.1016/j.gca.2004.05.031
Reedy OC, Jardine PM, Wilson GV, Selim HM (1996) Quantifying the diffuse mass transfer of nonreactive solute in columns of fractured saprolite using flow interruption. Soil Sci Soc Am J 60:1376–1384
Reich T, Moll H, Arnold T, Denecke MA, Henning C, Geipel G et al (1998) An EXAFS study of uranium(VI) sorption onto silica gel and ferrihydrite. J Electron Spectrosc Relat Phenom 96:237–243. doi:10.1016/S0368-2048(98)00242-4
Riley RG, Zachara JM, Wobber FJ (1992) Chemical contaminants on DOE lands and selection of contaminant mixtures for subsurface science research; DOE/ER-0547T DOE/ER-0547T. U.S. Department of Energy, Office of Energy Research, Washington, DC
Rosentreter JJ, Quarder HS, Smith RW, McLing T (1998) Uranium sorption onto natural sands as a function of sediment characteristics and solution pH. In: Jenne EA (ed) Adsorption of metals by geomedia. Academic Press, San Diego, pp 181–208
Rovira M, El Aamrani FZ, Duro L, Casas I, de Pablo J, Brouno J et al (2000) Experimental study and modeling of uranium(VI) transport through ferrous olivine rock columns. Radiochim Acta 88:6665–6671. doi:10.1524/ract.2000.88.9-11.665
Rovira M, El Aamrani S, Duro L, Gimenez J, de Pablo J, Bruno J (2007) Interaction of uranium with in situ anoxically generated magnetite on steel. J Hazard Mater 147:726–731. doi:10.1016/j.jhazmat.2007.01.067
Savenko AV (2001) Sorption of UO2 2+ on calcium carbonate. Radiochemistry 43:193–196. doi:10.1023/A:1012827617901
Schulze DG (2002) An introduction of soil mineralogy. In: Dixon JB, Schulze DG (eds) Soil mineralogy with environmental applications. Soil Science Society of America, Inc., Madison, pp 1–36
Schwertmann U, Taylor RM (1989) Iron oxides. In: Dixon JB, Weed SB (eds) Minerals in soil environments. Soil Science Society of America, Madison
Serne RJ, Schaef HT, Bjornstad BN, Williams BA, Lanigan DC, Horton DG et al (2001) Characterization of uncontaminated sediments from the Hanford Reservation-RCRA borehole core and composite samples PNNL-2001-1. Pacific Northwest National Laboratory, Richland
Serne JN, Brown CF, Schaef HT, Pierce EM, Lindberg MJ, Wang Z et al (2002) 300 Area uranium leach and adsorption project PNNL-14022. Pacific Northwest National Laboratory, Richland
Serne JN, Bjornstad BN, Horton DG, Lanigan DC, Lindenmeier CW, Lindberg JW et al (2004) Characterization of vadose zone sediments below the TX Tank Farm: Boreholes C3830, C3831, C3832 and RCRA borehole 299-W10-27. Report Number PNNL-14594. Pacific Northwest National Laboratory, Richland, WA
Sposito G (1984) The surface chemistry of soils. Oxford University Press, New York
Sposito G (1989) The chemistry of soils. Oxford University Press, New York
Sposito G (2004) The surface chemistry of natural particles. Oxford University Press, New York
Steefel CI, Van Cappellen P (1998) Reactive transport modeling of natural systems––Preface. J Hydrol (Amst) 209:1–7. doi:10.1016/S0022-1694(98)00182-6
Steefel CI, DePaolo DJ, Lichtner PC (2005) Reactive transport modeling: an essential tool and a new research approach for the Earth sciences. Earth Planet Sci Lett 240:539–558. doi:10.1016/j.epsl.2005.09.017
Strathmann TJ, Stone AT (2003) Mineral surface catalysis of reactions between Fe-II and oxime carbamate pesticides. Geochim Cosmochim Acta 67:2775–2791. doi:10.1016/S0016-7037(03)00088-7
Stumm W (1992) Chemistry of the solid-water interface. Processes at the mineral-water and particle-water interface in natural systems. Wiley, New York
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York
Sylwester ER, Hudson EA, Allen PG (2000) The structure of uranium(VI) sorption complexes on silica, alumina, and montmorillonite. Geochim Cosmochim Acta 64:2431–2438. doi:10.1016/S0016-7037(00)00376-8
Szecsody JE, Cantrell KJ, Krupka KM, Resch CT, Williams MD, Fruchter JS (1998) Uranium mobility during in situ redox manipulation of the 100 area of the Hanford site. PNNL-12048-UC-2000. Pacific Northwest National Laboratory, Richland, WA
Todorov PT, Ilieva EN (2006) Contamination with uranium from natural and anthropological sources. Rom J Phys 51:27–34
Tripathi VS (1983) Uranium transport modeling: geochemical data and sub-models. Stanford University, Stanford
Ulrich KU, Rossberg A, Foerstendorf H, Zanker H, Scheinost AC (2006) Molecular characterization of uranium(VI) sorption complexes on iron(III)-rich acid mine water colloids. Geochim Cosmochim Acta 70:5469–5487. doi:10.1016/j.gca.2006.08.031
Um W, Serne RJ, Brown CF, Last GV (2007a) U(VI) adsorption on aquifer sediments at the Hanford Site. J Contam Hydrol 93:255–269. doi:10.1016/j.jconhyd.2007.03.002
Um W, Mattigod S, Serne RJ, Fryxell GE, Kim DH, Troyer LD (2007b) Synthesis of nanoporous zirconium oxophosphate and application for removal of U(VI). Water Res 41:3217–3226. doi:10.1016/j.watres.2007.05.030
van Geen A, Robertson AP, Leckie JO (1994) Complexation of carbonate species at the goethite surface: implications for adsorption of metal ions in natural waters. Geochim Cosmochim Acta 58:2073–2086. doi:10.1016/0016-7037(94)90286-0
van Genuchten MT, Wierenga PJ (1976) Mass transfer studies in sorbing porous media: I. Analytical solutions. Soil Sci Soc Am J 40:473–480
van Genuchten MT, Wierenga PJ (1977) Mass transfer studies in sorbing porous media. II. Experimental evaluation with tritium-labeled water. Soil Sci Soc Am J 41:272–278
Villalobos M, Trotz MA, Leckie JO (2001) Surface complexation modeling of carbonate effects on the adsorption of Cr(VI), Pb(II) and U(VI) on goethite. Environ Sci Technol 35:3849–3856. doi:10.1021/es001748k
Waite TD, Davis JA, Payne TE, Waychunas GA, Xu N (1994) Uranium(VI) adsorption to ferrihydrite: application of a surface complexation model. Geochim Cosmochim Acta 58:5465–5478. doi:10.1016/0016-7037(94)90243-7
Waite TD, Davis JA, Fenton BR, Payne TE (2000) Approaches to modelling uranium(VI) adsorption on natural mineral assemblages. Radiochim Acta 88:687–693. doi:10.1524/ract.2000.88.9-11.687
Wang S, Jaffe PR, Li G, Wang SW, Rabitz HA (2003) Simulating bioremediation of uranium-contaminated aquifers; uncertainty assessment of model parameters. J Contam Hydrol 64:283–307. doi:10.1016/S0169-7722(02)00230-9
Wang Z, Zachara JM, Yantasee W, Gassman PL, Liu C, Joly AG (2004) Cryogenic laser induced fluorescence characterization of U(VI) in Hanford vadose zone pore waters. Environ Sci Technol 38:5591–5597. doi:10.1021/es049512u
Wang Z, Zachara JM, McKinley JP, Smith SC (2005) Cryogenic laser induced U(VI) fluorescence studies of the U(VI) substituted natural calcite: implications to U(VI) speciation in contaminated Hanford sediments. Environ Sci Technol 39:2651–2659. doi:10.1021/es048448d
Wazne M, Korfiatis GP, Meng XG (2003) Carbonate effects on hexavalent uranium adsorption by iron oxyhydroxide. Environ Sci Technol 37:3619–3624. doi:10.1021/es034166m
Wazne M, Meng XG, Korfiatis GP, Christodoulatos C (2006) Carbonate effects on hexavalent uranium removal from water by nanocrystalline titanium dioxide. J Hazard Mater 136:47–52. doi:10.1016/j.jhazmat.2005.11.010
Webb SM, Fuller CC, Tebo BM, Bargar JR (2006) Determination of uranyl incorporation into biogenic manganese oxides using X-ray absorption spectroscopy and scattering. Environ Sci Technol 40:771–777. doi:10.1021/es051679f
Williams AGB, Scherrer MM (2004) Spectroscopic evidence for Fe(II)–Fe(III) electron transfer at the iron oxide–water interface. Environ Sci Technol 38:4782–4790. doi:10.1021/es049373g
Yabusaki SB, Fang Y, Long PE, Resch CT, Peacock AD, Komlos J et al (2007) Uranium removal from groundwater via in situ biostimulation: field-scale modeling of transport and biological processes. J Contam Hydrol 93:216–235. doi:10.1016/j.jconhyd.2007.02.005
Zachara JM, Davis JA, Liu C, McKinley JP, Qafoku NP, Wellman DM, Yabusaki SB (2005) Uranium geochemistry in vadose zone and aquifer sediments from the 300 Area uranium plume PNNL-15121
Zheng Z, Tokunaga TK, Wan J (2003) Influence of calcium carbonate on U(VI) sorption to soils. Environ Sci Technol 37:5603–5608. doi:10.1021/es0304897
Zinn B, Harvey CF (2003) When good statistical models of aquifer heterogeneity go bad: a comparison of flow, dispersion, and mass transfer in connected and multivariate Gaussian hydraulic conductivity fields. Water Resour Res 39:1051. doi:10.1029/2001WR001146
Acknowledgments
This work was partially supported by the U.S. Department of Energy (DOE)––Environmental Remediation Sciences Program (ERSP), through Dr. Philip E. Long (Pricipal Investigator) IFC project, Rifle, Colorado, USA. Pacific Northwest National Laboratory is operated for the DOE by Battelle Memorial Institute under Contract DE-AC06-76RLO 1830.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qafoku, N.P., Icenhower, J.P. Interactions of aqueous U(VI) with soil minerals in slightly alkaline natural systems. Rev Environ Sci Biotechnol 7, 355–380 (2008). https://doi.org/10.1007/s11157-008-9137-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-008-9137-8