Abstract
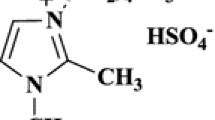
We report a new, one-pot, efficient, four-component condensation of hydrazine hydrate, ethyl acetoacetate, arylaldehydes, and malononitrile in the presence of a reusable weakly basic ionic liquid, 1,8-diazabicyclo[5.4.0]-undec-7-en-8-ium acetate, as catalyst, for synthesis of 6-amino-4-aryl-5-cyano-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole derivatives under solvent-free conditions at room temperature. The method has several advantages, for example good yields, short reaction times, and simple work-up procedure.




Similar content being viewed by others
References
A.M. Inamuddin, Green Solvents I: Properties and Applications of Ionic Liquids (Springer, United Kingdom, 2012)
A.M. Inamuddin, Green Solvents II: Properties and Applications of Ionic Liquids (Springer, United Kingdom, 2012)
P. Tundo, A. Perosa, F. Zecchini, Methods and Reagents for Green Chemistry: An Introduction (Wiley, New Jersey, 2007)
P. Wasserscheid, P. Welton, Ionic Liquids in Synthesis (Wiley–VCH, Germany, 2003)
C. Montagne, J.J. Shiers, M. Shipman, Tetrahedron Lett. 47, 9207 (2006)
H. Bienayme, Multicomponent Reactions (Wiley–VCH, Weinheim, 2005)
S. Bräse, C. Gil, K. Knepper, Bioorg. Med. Chem. 10, 2415 (2002)
H.C. Hailes, Org. Process Res. Dev. 11, 114 (2007)
E.S. El-Tamany, F.A. El-Shahed, B.H. Mohamed, J. Serb. Chem. Soc. 64, 9 (1999)
Z.H. Ismail, G.M. Aly, M.S. El-Degwi, H.I. Heiba, M.M. Ghorab, Egypt J. Biotechnol. 13, 73 (2003)
M.E.A. Zaki, H.A. Soliman, O.A. Hiekal, A.E.Z. Rashad, Naturforsch. C 61, 1 (2006)
F.M. Abdelrazek, P. Metz, N.H. Metwally, S.F. El-Mahrouky, Arch. Pharm. 339, 456 (2006)
F.M. Abdelrazek, P. Metz, O. Kataeva, A. Jaeger, S.F. El-Mahrouky, Arch. Pharm. 340, 543 (2007)
A. Kimata, H. Nakagawa, R. Ohyama, T. Fukuuchi, S. Ohta, T. Suzuki, N. Miyata, J. Med. Chem. 50, 5053 (2007)
V.Y. Sosnovskikh, M.A. Barabanov, B.I. Usachev, R.A. Irgashev, V.S. Moshkin, Russ. Chem. Bull. Int. Ed. 54, 2846 (2005)
H. Wamhoff, E. Kroth, K. Strauch, Synthesis 11, 1129 (1993)
L.A. Rodinovskaya, A.V. Gromova, A.M. Shestopalov, N. Nesterov, Russ. Chem. Bull. Int. Ed. 52, 2207 (2003)
H.R. Shaterian, A. Hosseinian, M. Ghashang, Tetrahedron Lett. 49, 5804 (2008)
H.R. Shaterian, M. Ranjbar, K. Azizi, J. Mol. Liq. 162, 95 (2011)
H.R. Shaterian, M. Ranjbar, J. Mol. Liq. 160, 40 (2011)
A.-G. Ying, L. Liu, G.-F. Wu, G. Chen, X.-Z. Chen, W.-D. Ye, Tetrahedron Lett. 50, 1653 (2009)
M. Babaie, H. Sheibani, Arab. J. Chem. 4, 159 (2011)
H. Mecadon, M.R. Rohman, M. Rajbangshi, B. Myrboh, Tetrahedron Lett. 52, 2523 (2011)
G. Vasuki, K. Kumaravel, Tetrahedron Lett. 49, 5636 (2008)
K. Kanagaraj, K. Pitchumani, Tetrahedron Lett. 51, 3312 (2010)
A. Siddekhab, A. Nizama, M.A. Pashaa, Spectrochim. Acta. A. 81, 431 (2011)
Y.M. Litvinov, A.A. Shestopalov, L.A. Rodinovskaya, A.M. Shestopalov, J. Comb. Chem. 11, 914 (2009)
Acknowledgments
We are grateful to the University of Sistan and Baluchestan Research Council for partial support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaterian, H.R., Sedghipour, M. & Mollashahi, E. [DBU][Ac]-catalyzed mild preparation of 6-amino-4-aryl-5-cyano-3-methyl-1,4-dihydropyrano[2,3-c]pyrazole derivatives. Res Chem Intermed 40, 2721–2728 (2014). https://doi.org/10.1007/s11164-013-1124-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-013-1124-1