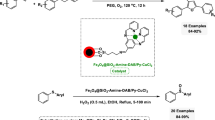
Abstract
Ni2B@Cu2O and Ni2B@CuCl2 are introduced as simple and efficient earth-abundant transition-metal-based nanocomposites for the green one-pot reductive acetylation of aromatic nitro compounds and direct N-acetylation of arylamines using a solvent-free mechanochemical grinding technique. The designed Ni2B-based nanocomposites were characterized by Fourier-transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD) analysis, and scanning electron microscopy (SEM) with energy-dispersive X-ray (EDX) spectroscopy. Notable advantages of these methods include broad substrate scope, use of a solvent-free mechanochemical grinding technique, implementation of earth-abundant transition-metal-based nanocomposites as simple and practical catalysts, and short reaction time and high yield at ambient condition. The mentioned methods can also be successfully applied for the synthesis of a broad range of other amides (especially substituted acetamides) using green chemistry protocols. Also, the recoverability and reusability of the mentioned new nanocomposites were investigated.
Graphical abstract










Similar content being viewed by others
References
M.G. Dekamin, M. Azimoshan, L. Ramezani, Green Chem. 15, 811 (2013)
A.L. Garay, A. Pichon, S.L. James, Chem. Soc. Rev. 36, 846 (2007)
K. Nikoofar, Z. Khademi, Res. Chem. Intermed. 42, 3929 (2016)
M. Rimaz, J. Khalafy, H. Mousavi, Res. Chem. Intermed. 42, 8185 (2016)
M. Rimaz, J. Khalafy, H. Mousavi, S. Bohlooli, B. Khalili, J. Heterocycl. Chem. 54, 3174 (2017)
A. Kumar, M.S. Rao, I. Ahmad, B. Khungar, Aust. J. Chem. 62, 322 (2009)
M. Rimaz, Z. Jalalian, H. Mousavi, R.H. Prager, Tetrahedron Lett. 57, 105 (2016)
M. Rimaz, H. Mousavi, P. Keshavarz, B. Khalili, Curr. Chem. Lett. 4, 159 (2015)
S. Zhaleh, N. Hazeri, M.R. Faghihi, M.T. Maghsoodlou, Res. Chem. Intermed. 42, 8069 (2016)
M. Rimaz, H. Mousavi, M. Behnam, B. Khalili, Curr. Chem. Lett. 5, 145 (2016)
M.G. Dekamin, M. Eslami, Green Chem. 16, 4914 (2014)
J.-B. Yu, G. Peng, Z.-J. Jiang, Z.-K. Hong, W.-K. Su, Eur. J. Org. Chem. 32, 5340 (2016)
X. Chen, H. Yang, Z. Zhong, N. Yan, Green Chem. 19, 2783 (2017)
I. Dokli, M. Gredičak, Eur. J. Org. Chem. 12, 2727 (2015)
A. Sarkar, S. Santra, S.K. Kundu, A. Hajra, G.V. Zyryanov, O.N. Chupakhin, V.C. Charushin, A. Majee, Green Chem. 18, 4475 (2016)
H. Sharma, M. Singh, D.O. Jang, Green Chem. 16, 4922 (2014)
M. Vadivelu, S. Sugirdha, P. Dheenkumar, Y. Arun, K. Karthikeyan, C. Praveen, Green Chem. 19, 3601 (2017)
X. Zhu, Z. Li, C. Jin, L. Xu, Q. Wu, W. Su, Green Chem. 11, 163 (2009)
R. Teimuri-Mofrad, A. Shahrisa, M. Gholamhosseini-Nazari, N. Arsalani, Res. Chem. Intermed. 42, 3425 (2016)
N. Ghaffari Khaligh, H.S. Abbo, S.J.J. Titinchi, Res. Chem. Intermed. 43, 901 (2017)
Z.T. Bhutia, G. Prasannakumar, A. Das, M. Biswas, A. Chatterjee, M. Banerjee, ChemistrySelect 2, 1183 (2017)
Q.-S. Ding, J.-L. Zhang, J.-X. Chen, M.-C. Liu, J.-C. Ding, H.-Y. Wu, J. Heterocycl. Chem. 49, 375 (2012)
S. Kumar, P. Sharma, K.K. Kapoor, M.S. Hundal, Tetrahedron 64, 356 (2008)
S. Kuntikana, C. Bhat, M. Kongot, S.I. Bhat, A. Kumar, ChemistrySelect 1, 1723 (2016)
J. Li, D.-N. Jiang, J.-X. Chen, M.-C. Liu, J.-C. Ding, H.-Y. Wu, J. Heterocycl. Chem. 48, 403 (2011)
D. Sharma, Res. Chem. Intermed. 41, 927 (2015)
A.K. Bose, S. Pednekar, S.N. Ganguly, G. Chakraborty, M.S. Manhas, Tetrahedron Lett. 45, 8351 (2004)
Y. Zha, Z. Meng, M. Wang, M. Gu, C. Li, P. Cai, L. Rong, Res. Chem. Intermed. 42, 1217 (2016)
H. Naeimi, H. Foroughi, Res. Chem. Intermed. 42, 3999 (2016)
B. Karimi, F. Mansouri, H.M. Mirzaei, ChemCatChem 7, 1736 (2015)
B. Karimi, F. Mansouri, H. Vali, Green Chem. 16, 2587 (2014)
M.-C. Daniel, D. Astruc, Chem. Rev. 104, 293 (2004)
P. Paul, P. Bhanja, N. Salam, U. Mandi, A. Bhaumik, S.A. Alam, S.M. Islam, J. Colloid Interface Sci. 493, 206 (2017)
P. Hervés, M. Pérez-Lorenzo, L.M. Liz-Marzán, J. Dzubiella, Y. Lu, M. Ballauff, Chem. Soc. Rev. 41, 5577 (2012)
A. Khazaei, M. Khazaei, M. Nasrollahzadeh, Tetrahedron 73, 5624 (2017)
D. Elhamifar, P. Mofatehnia, M. Faal, J. Colloid Interface Sci. 504, 268 (2017)
M.B. Gawande, A. Goswami, T. Asefa, H. Guo, A.V. Biradar, D.-L. Peng, R. Zboril, R.S. Varma, Chem. Soc. Rev. 44, 7540 (2015)
J. Rakhtshah, S. Salehzadeh, Res. Chem. Intermed. 43, 6973 (2017)
M. Ghashang, S. Guhanathan, S.S. Mansoor, Res. Chem. Intermed. 43, 7257 (2017)
S.P. Kunde, K.G. Kanade, B.K. Karale, H.N. Akolkar, P.V. Randhavane, Res. Chem. Intermed. 43, 7277 (2017)
D. Wang, D. Astruc, Chem. Soc. Rev. 46, 816 (2017)
M.B. Gawande, A. Goswami, F.-X. Felpin, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zboril, R.S. Varma, Chem. Rev. 116, 3722 (2016)
V.K. Akkilagunta, R.R. Kakulapati, J. Org. Chem. 76, 6819 (2011)
T. Tamoradi, B. Mehraban-Esfandiari, M. Ghadermarzi, A. Ghorbani-Choghamarani, Res. Chem. Intermed. 44, 1363 (2018)
S. Asghari, M. Mohammadnia, Res. Chem. Intermed. 43, 7193 (2017)
N. Basavegowda, K.B.S. Magar, K. Mishra, Y.R. Lee, New J. Chem. 38, 5415 (2014)
D. Cantillo, M. Baghbanzadeh, C.O. Kappe, Angew. Chem. Int. Ed. 51, 10190 (2012)
R. Faraghi-Alamdari, N. Zekri, F. Mansouri, Res. Chem. Intermed. 43, 6537 (2017)
M. Gholinejad, N. Jeddi, ACS Sustain. Chem. Eng. 2, 2658 (2014)
H. Sharghi, R. Khalifeh, M.M. Doroodmand, Adv. Synth. Catal. 351, 207 (2009)
G. Molteni, C.L. Bianchi, G. Marinoni, N. Santo, A. Ponti, New J. Chem. 30, 1137 (2006)
J. Seo, D. Cha, K. Takanabe, J. Kubota, K. Domen, ACS Catal. 3, 2181 (2013)
B. Amirheidari, M. Seifi, M. Abaszadeh, Res. Chem. Intermed. 42, 3413 (2016)
A. Dömling, Chem. Rev. 106, 17 (2006)
N.G. Shabalala, S. Maddila, S.B. Jonnalagadda, Res. Chem. Intermed. 42, 8097 (2016)
M. Rimaz, H. Mousavi, Turk. J. Chem. 37, 252 (2013)
M. Rimaz, H. Mousavi, L. Nikpey, B. Khalili, Res. Chem. Intermed. 43, 3925 (2017)
M. Rimaz, H. Mousavi, M. Behnam, L. Sarvari, B. Khalili, Curr. Chem. Lett. 6, 55 (2016)
S. Golchin, M.H. Mosslemin, A. Yazdani-Elah-Abadi, N. Shams, Res. Chem. Intermed. 43, 1735 (2017)
N. Azizi, M. Edrisi, Res. Chem. Intermed. 43, 379 (2017)
M. Abbasi, Res. Chem. Intermed. 42, 3303 (2016)
A. Farhadi, J. Noei, R.H. Aliyari, M. Albakhtiyari, M.A. Takassi, Res. Chem. Intermed. 42, 1401 (2016)
G. Khanna, P. Saluja, J.M. Khurana, Aust. J. Chem. 70, 1285 (2017)
A. Mohammadinezhad, B. Akhlaghinia, Aust. J. Chem. 71, 32 (2018)
M. Rimaz, H. Mousavi, B. Khalili, F. Aali, J. Chin. Chem. Soc. (2018). https://doi.org/10.1002/jccs.201700470
M. Rimaz, B. Khalili, G. Khatyal, H. Mousavi, F. Aali, Aust. J. Chem. 70, 1274 (2017)
P. Wang, J. Xia, Y. Gu, Tetrahedron Lett. 56, 7120 (2015)
S.S. Kotha, S. Badigenchala, G. Sekar, Adv. Synth. Catal. 357, 1437 (2015)
Z. Fu, J. Lee, B. Kang, S.H. Hong, Org. Lett. 14, 6028 (2012)
J. Pan, N.O. Devarie-Baez, M. Xian, Org. Lett. 13, 1092 (2011)
T.F. Molinki, Org. Biomol. Chem. 16, 21 (2018)
S. Yao, Y. Qian, ChemistrySelect 3, 12367 (2018)
K.V. Sashidhara, G.R. Palnati, R.P. Dodda, R. Sonkar, A.K. Khanna, G. Bhatia, Eur. J. Med. Chem. 57, 302 (2012)
M. Bhat, S.L. Belegari, N.K.H. Kumar, S.M. Kumar, Res. Chem. Intermed. 43, 361 (2017)
S. Zhu, S. Xu, Z. Zhao, J. Jiang, Res. Chem. Intermed. 43, 3415 (2017)
C. De Risi, G.P. Pollini, V. Zanirato, Chem. Rev. 116, 3241 (2016)
R.M. de Figueiredo, J.-S. Suppo, J.-M. Campagne, Chem. Rev. 116, 12029 (2016)
X. Guo, A. Facchetti, T.J. Marks, Chem. Rev. 114, 8943 (2014)
R. Ningegowda, S. Bhaskaran, A.M. Sajith, C. Aswathanarayanappa, M.S.A. Padusha, N.S. Shivananju, B.S. Priya, Aust. J. Chem. 70, 44 (2017)
C. Urda, R. Fernández, J. Rodríguez, M. Pérez, C. Jiménez, C. Cuevas, J. Nat. Prod. 80, 3054 (2017)
F.H. Al-Awadhi, B.K. Law, V.J. Paul, H. Luesch, J. Nat. Prod. 80, 2969 (2017)
B. Zeynizadeh, F. Sepehraddin, J. Organomet. Chem. 70, 856 (2018)
S. Karami, B. Zeynizadeh, Z. Shokri, Cellulose 25, 3229 (2018)
Z. Shokri, B. Zeynizadeh, S.A. Hosseini, J. Colloid Interface Sci. 485, 99 (2017)
B. Zeynizadeh, I. Mohammadzadeh, Z. Shokri, S.A. Hossein, J. Colloid Interface Sci. 500, 285 (2017)
Z. Shokri, B. Zeynizadeh, S.A. Hosseini, B. Azizi, J. Iran. Chem. Soc. 14, 101 (2017)
Z. Shokri, B. Zeynizadeh, J. Iran. Chem. Soc. 14, 2467 (2017)
B. Zeynizadeh, F. Sepehraddin, J. Iran. Chem. Soc. 14, 2649 (2017)
B. Zeynizadeh, M. Zabihzadeh, Z. Shokri, J. Iran. Chem. Soc. 13, 1487 (2016)
C.A. Brown, H.C. Brown, J. Am. Chem. Soc. 85, 1003 (1963)
F. Taghavi, C. Falamaki, A. Shabanov, L. Bayrami, A. Roumianfar, Appl. Catal. A Gen. 407, 173 (2011)
G. Ranjani, R. Nagarajan, Org. Lett. 19, 3974 (2017)
X. Zhang, Y. Zhang, H. Huang, J. Cai, K. Ding, S. Lin, New J. Chem. 42, 458 (2018)
A.L. Gajengi, C.S. Fernandes, B.M. Bhanage, Mol. Catal. 451, 13 (2017)
S. Meghana, P. Kabra, S. Chakraborty, N. Padmavathy, RSC Adv. 5, 12293 (2015)
C. Qi, J. Zheng, Electroanalysis 28, 477 (2016)
M. Salavati-Niasari, F. Davar, Mater. Lett. 63, 441 (2009)
W. Wang, Y. Tu, P. Zhang, G. Zhang, CrystEngComm 13, 1838 (2011)
W. Wang, Y. Tu, L. Wang, Y. Liang, H. Shi, Appl. Surf. Sci. 264, 399 (2013)
T. Jiang, T. Xie, L. Chen, Z. Fu, D. Wang, Nanoscale 5, 2938 (2013)
Y. Bai, W. Zhang, Z. Zhang, J. Zhou, X. Wang, C. Wang, W. Huang, J. Jiang, Y. Xiong, J. Am. Chem. Soc. 136, 14650 (2014)
R.N. Baruah, Indian J. Chem. 39B, 300 (2000)
K.Y. Lee, J.M. Kim, J.N. Kim, Bull. Korean Chem. Soc. 23, 1355 (2002)
B.H. Kim, R. Han, F. Piao, Y.M. Jun, W. Baik, B.M. Lee, Tetrahedron Lett. 44, 77 (2003)
X. Wang, H. Guo, G. Xie, Y. Zhang, Synth. Commun. 34, 3001 (2004)
Y.-S. Jia, Q. Li, X.-H. Wang, H.L. Wang, X.-T. Liu, J. Shanghai Univ. 10, 277 (2006)
A. Bhattacharya, V.C. Purohit, V. Suarez, R. Tichkule, G. Parmer, F. Rinaldi, Tetrahedron Lett. 47, 1861 (2006)
M.L. Kantam, R.S. Reddy, K. Srinivas, R. Chakravarti, B. Sreedhar, F. Figueeras, C.V. Reddy, J. Mol. Catal. A Chem. 355, 96 (2012)
M. Ghaffarzadeh, P. Akhavan, Chem. Lett. 43, 1417 (2014)
M. Hosseini-Sarvari, Z. Razmi, Appl. Surf. Sci. 324, 265 (2015)
Acknowledgements
Financial support of this work by the Research Council of Urmia University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zeynizadeh, B., Younesi, R. & Mousavi, H. Ni2B@Cu2O and Ni2B@CuCl2: two new simple and efficient nanocatalysts for the green one-pot reductive acetylation of nitroarenes and direct N-acetylation of arylamines using solvent-free mechanochemical grinding. Res Chem Intermed 44, 7331–7352 (2018). https://doi.org/10.1007/s11164-018-3559-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3559-x