Abstract
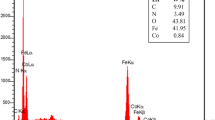
Grafting of 4-(sulfoamino)butanoic acid on superparamagnetic γ-Fe2O3@SiO2 nanoparticles afforded γ-Fe2O3@SiO2@4-(sulfoamino)butanoic acid as a novel heterogeneous nanocatalyst, which was characterized by X-ray diffraction, Fourier transform infrared spectroscopy, vibrating sample magnetometry, field emission scanning electron microscopy, and thermal gravimetric analysis. In this research, we report a convenient and one-pot efficient direct protocol for the pseudo four-component preparation of 5-(aryl)-5H-spiro[diindeno[1,2-b:2′,1′-e]pyridine-11,3′-indoline]-2′,10,12-trione derivatives via cascade condensation reaction of 1,3-indandione and isatins with various aromatic amine in the presence of the catalytic amount of γ-Fe2O3@SiO2@4-(sulfoamino)butanoic acid under green conditions in aqueous media. This procedure offers several advantages such as: very easy reaction conditions, simple work-up, or purification, excellent yields, high purity of the desired product, atom economy, and short reaction times. The superparamagnetic catalyst is magnetically separable and retained chemical stability after recycling for at least five consecutive runs without detectable activity loss.
Graphical abstract












Similar content being viewed by others
References
L. Wu, A. Mendoza-Garcia, Q. Li, S. Sun, Chem. Rev. 116, 10473 (2016)
A.S. Paulus, R. Heinzler, H.W. Ooi, M. Franzreb, ACS Appl. Mater. Interfaces 7, 14279 (2015)
A.P. Tiwari, S.J. Ghosh, S.H. Pawar, Anal. Methods 7, 10109 (2015)
Q. Luo, X. Xiao, X. Dai, Z. Duan, D. Pan, H. Zhu, X. Li, L. Sun, K. Luo, Q. Gong, ACS Appl. Mater. Interfaces. 10, 1575 (2018)
S.V. Sokolov, E. Kätelhön, R.G. Compton, J. Phys. Chem. C 119, 25093 (2015)
M.F. Silva, A.A.W. Hechenleitner, J.M. Irache, A.J.A. de Oliveira, E.A. Gómez Pineda, Mater. Res. 18, 1400 (2015)
X. Yao, J. Jing, F. Liang, Z. Yang, Macromolecules 49, 9618 (2016)
A. Taghvimi, H. Hamishehkar, J. Chromatogr. B 1041–1042, 113 (2017)
S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L.V. Elst, R.N. Muller, Chem. Rev. 108, 2064 (2008)
R.K. Sharma, M. Yadav, M.B. Gawande, in ACS Symp. Ser. 2016, p. 1
N. Pal, A. Bhaumik, RSC Adv. 5, 24363 (2015)
N. Taheri, F. Heidarizadeh, A. Kiasat, J. Magn. Magn. Mater. 428, 481 (2017)
M. Abdollahi-Alibeik, A. Rezaeipoor-Anari, J. Magn. Magn. Mater. 398, 205 (2016)
W. Wu, C.Z. Jiang, V.A.L. Roy, Nanoscale 8, 9421 (2016)
P. Saravanan, K. Jayamoorthy, S. Anandakumar, J. Lumin. 178, 241 (2016)
D.G. Wang, C. Deraedt, J. Ruiz, D. Astruc, Acc. Chem. Res. 48, 1871 (2016)
M. Zhang, Y.H. Liu, Z.R. Shang, H.C. Hu, Z.H. Zhang, Catal. Commun. 88, 39 (2017)
M. Zhang, P. Liu, Y.H. Liu, Z.R. Shang, H.C. Hu, Z.H. Zhang, RSC Adv. 6, 106160 (2016)
K.M. Ho, P. Li, Langmuir 24, 1801 (2008)
K. Azizi, A. Heydari, RSC Adv. 4, 8812 (2008)
K. Tsuji, J. Injuk, R.V. Grieken, X-Ray Spectrometry: Recent Technological Advances (Wiley, Hoboken, 2005)
D.R. Chandam, A.G. Mulik, D.R. Patil, A.P. Patravale, D.R. Kumbhar, M.B. Deshmukh, J. Mol. Liq. 219, 573 (2016)
J. Sindhu, H. Singh, M. Khurana, Synth. Commun. 45, 202 (2015)
R. Ghahremanzadeh, G. Imani Shakibaei, S. Ahadi, A. Bazgir, J. Comb. Chem. 12, 191 (2010)
Acknowledgements
We are grateful for the financial support from the Research Council of University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi, H., Shaterian, H.R. γ-Fe2O3@SiO2@4-(sulfoamino)butanoic acid as a novel superparamagnetic nanocatalyst promoted green synthesis of 5-(aryl)-5H-spiro[diindeno[1,2-b:2′,1′-e]pyridine-11,3′-indoline]-2′,10,12-trione derivatives. Res Chem Intermed 44, 7519–7538 (2018). https://doi.org/10.1007/s11164-018-3571-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3571-1