Abstract
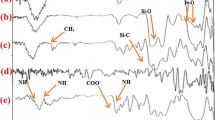
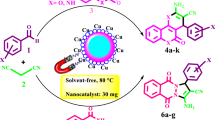
Nano-Fe3O4@SiO2-3,4-dihydroxybenzaldehyde/barbituric acid/phthalhydrazide-FeCl3 (nano-Fe3O4@SiO2-HBP-FeCl3) was prepared and authenticated by usual analytical and spectroscopic techniques. The prepared nano-Fe3O4@SiO2-HBP-FeCl3 was applied to acridinedione and spiroquinazolin-4(3H)-one derivatives under green conditions. In addition, this research offers several advantages such as very easy reaction conditions, simple work-up, excellent yields, high purity of the desired product, short reaction time and one-pot reaction to synthesize Fe3O4@SiO2-HBP-FeCl3. The recycling studies revealed that catalyst could be easily recovered using an external magnet and reused five times without significant loss of its catalytic activity.











Similar content being viewed by others
References
Z. Nasresfahani, M.Z. Kassaee, E. Eidi, Appl. Organomet. Chem. 31, e3800 (2017)
P. Kumar, K. Kadyan, M. Duhan, J. Sindhu, K. Hussain, S. Lal, Chem. Pap. 73, 1153 (2019)
R.K. Sharma, M. Yadav, M.B. Gawande, ACS Symp. Ser. 1238, 1 (2016)
S. Gandhi, N. Nagalakshmi, I. Baskaran, V. Dhanalakshmi, M.R. Gopinathan, N.R. Anbarasan, J. Appl. Polym. Sci. 118, 1666 (2010)
T. Demirci, B. Çelik, Y. Yıldız, S. Eriş, M. Arslan, F. Sen, B. Kilbas, RSC Adv. 6, 76948 (2016)
E. Esma, Y. Yunus, K. Benan, Ş. Fatih, J. Nanosci. Nanotechnol. 16, 5944 (2016)
F. Şen, G. Gökağaç, S. Şen, J. Nanopart. Res. 15, 1979 (2013)
R. Ayranci, G. Baskaya, M. Guzel, S. Bozkurt, M. Ak, A. Savk, F. Sen, Nano-Struct. Nano-Objects 11, 13 (2017)
Y. Yildiz, T.O. Okyay, B. Sen, B. Gezer, S. Kuzu, A. Savk, E. Demir, Z. Dasdelen, H. Sert, F. Sen, ChemistrySelect 2, 697 (2017)
Z. Tai, M.A. Isaacs, L.J. Durndell, C.M.A. Parlett, A.F. Lee, K. Wilson, Mol. Catal. 449, 137 (2018)
A. Mirhashemi, M.A. Amrollahi, Res. Chem. Intermed. 45, 2549 (2019)
M.M. Khodaei, A. Alizadeh, M. Haghipour, Res. Chem. Intermed. 45, 2727 (2019)
M. Gholamhosseini-Nazari, S. Esmati, K.D. Safa, A. Khataee, R. Teimuri-Mofrad, Res. Chem. Intermed. 45, 1841 (2019)
S.V. Sokolov, E. Kätelhön, R.G. Compton, J. Phys. Chem. C 119, 25093 (2015)
M.F. Silva, A.A.W. Hechenleitner, J.M. Irache, A.J.A. de Oliveira, E.A.G. Pineda, J. Mater. Sci. 18, 1400 (2015)
V. Polshettiwar, R.S. Varma, Green Chem. 12, 743 (2010)
R.N. Baig, R.S. Varma, Chem. Commun. 48, 6220 (2012)
M. Sheykhan, A. Yahyazadeh, L. Ramezani, Mol. Catal. 435, 166 (2017)
A. Bamoniri, N. Moshtael-Arani, RSC Adv. 5, 16911 (2015)
N. Azgomi, M. Mokhtar, J. Mol. Catal. A Chem. 398, 58 (2015)
M.A. Bodaghifard, S. Asadbegi, Z. Bahrami, J. Iran. Chem. 14, 365 (2017)
M.A. Zolfigol, M. Safaiee, N. Bahrami-Nejad, New J. Chem. 40, 5071 (2016)
F. Javaheri, S. Hassanajili, J. Appl. Polym. Sci. 133, 48 (2016)
A. Ghorbani-Choghamarani, G. Azadi, Appl. Organomet. Chem. 30, 247 (2016)
M.A. Zolfigol, M. Kiafar, M. Yarie, A.A. Taherpour, M. Saeidi-Rad, RSC Adv. 6, 50100 (2016)
Q. Dai, Y. Wang, W. Xu, Y. Liu, Y. Zhou, Mikrochim. Acta 184, 4433 (2017)
S.A. Hamrahian, J. Rakhtshah, S.M. Mousavi Davijani, S. Salehzadeh, Appl. Organomet. Chem. 10, e4501 (2018)
H.A. Patel, A.M. Sawant, V.J. Rao, A.L. Patel, A.V. Bedekar, Catal. Lett. 147, 2306 (2017)
D. Sahu, A.R. Silva, P. Das, RSC Adv. 5, 78553 (2015)
H. Singh, J.K. Rajput, G. Govil, P. Arora, J. Badhan, Appl. Organomet. Chem. 10, e4514 (2018)
M. Esmaeilpour, A.R. Sardarian, H. Firouzabadi, Appl. Organomet. Chem. 32, e4300 (2018)
A. Burak, P. Handan, K. Muharrem, S. Fatih, J. Nanosci. Nanotechnol. 16, 6498 (2016)
R. Ulus, Y. Yıldız, S. Eriş, B. Aday, F. Şen, ChemistrySelect 1, 3861 (2016)
X. Liu, Z. Ma, J. Xing, H. Liu, J. Magn. Magn. Mater. 270, 1 (2004)
V.R. Mudumala, R.R. Chinthaparthi, T. Yeon, Tetrahedron 70, 3762 (2014)
Z. Zarei, B. Akhlaghinia, New J. Chem. 41, 15485 (2017)
B. Aday, Y. Yıldız, R. Ulus, S. Eris, F. Sen, M. Kaya, New J. Chem. 40, 748 (2016)
H.R. Safaei, M. Safaei, M. Shekouhy, RSC Adv. 5, 6797 (2015)
A. Maleki, T. Kari, M. Aghaei, J. Porous Mater. 24, 1481 (2017)
X. Zhu, S.-R. Kang, L. Xia, J. Lee, N. Basavegowda, Y.-R. Lee, Mol. Divers. 19, 67 (2015)
K. Venkatesan, S.S. Pujari, K.V. Srinivasan, Synth. Commun. 39, 228 (2009)
B. Das, P. Thirupathi, I. Mahender, V. Saidi Reddy, Y.K. Rao, J. Mol. Catal. A Chem. 247, 233 (2006)
M. Dabiri, M. Baghbanzadeh, E. Arzroomchilar, Catal. Commun. 9, 939 (2008)
K. Niknam, F. Panahi, D. Saberi, M. Mohagheghnejad, J. Heterocycl. Chem. 47, 292 (2010)
M.M. Alam, A.T. Mubarak, M.A. Assiri, S.M. Ahmed, BMC Chem. 13, 40 (2019)
Acknowledgements
We gratefully appreciate the University of Sistan and Baluchestan Research Councils for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi, H., Shaterian, H.R. Ferric (III) complex supported on superparamagnetic Fe3O4@SiO2 as a reusable Lewis acid catalyst: a new highly efficient protocol for the synthesis of acridinedione and spiroquinazolin-4(3H)-one derivatives. Res Chem Intermed 46, 179–195 (2020). https://doi.org/10.1007/s11164-019-03942-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03942-w