Abstract
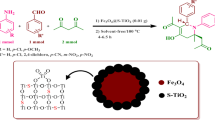
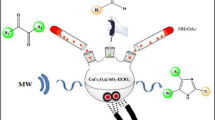
In this study, an efficient and novel procedure has been reported for loading sulfanilic acid on the surface of magnetite Fe3O4 nanoparticles using tris(hydroxymethyl) aminomethane and 1,2-dichloroethane. Next, the synthesized nanocatalyst was fully characterized using FT-IR, XRD, TGA, VSM, SEM, and TEM. The results show that the synthesis of magnetic nanocatalyst has been successful with a range of 2–20 nm in size. Finally, the catalytic activity of this superparamagnetic nanocatalyst was explored for the synthesis of tetrahydrobenzo[b]pyran and 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives. The synthesized nanocatalyst has advantages such as non-toxicity, short reaction time, easy workup, cleaner reaction profiles under mild reaction conditions.













Similar content being viewed by others
References
L. Chen, A. Noory Fajer, Z. Yessimbekov, M. Kazemi, M. Mohammadi, J. Sulfur Chem. 40, 451 (2019)
A. Ghorbani-Choghamarani, M. Mohammadi, Z. Taherinia, J. Iran. Chem. Soc. 16, 411 (2019)
A. Ghorbani-Choghamarani, M. Mohammadi, T. Tamoradi, M. Ghadermazi, Polyhedron 158, 25 (2019)
M. Kazemi, M. Ghobadi, A. Mirzaie, Nanotechnol. Rev. 7, 43 (2018)
M. Schulz‐Dobrick, I. Schnell, Open Chem. 3, SI1 (2007)
A. Ghorbani-Choghamarani, M. Mohammadi, R.H.E. Hudson, T. Tamoradi, Appl. Organomet. Chem. 33, e4977 (2019)
F. Chen, S. Xie, X. Huang, X. Qiu, J. Hazard. Mater. 322, 152 (2017)
J. Ke, J. Liu, H. Sun, H. Zhang, X. Duan, P. Liang, X. Li, M.O. Tade, S. Liu, S. Wang, Appl. Catal. B-Environ. 200, 47 (2017)
Z. Wen, Y. Zhang, Y. Wang, L. Li, R. Chen, Chem. Eng. J. 312, 39 (2017)
F. He, J. Luo, S. Liu, Chem. Eng. J. 294, 362 (2016)
Z.W. Seh, J. Kibsgaard, C.F. Dickens, I. Chorkendorff, J.K. Nørskov, T.F. Jaramillo, Science 355, eaad4998 (2017)
J. Shi, Chem. Rev. 113, 2139 (2013)
J. Wang, R. Nie, L. Xu, X. Lyu, X. Lu, Green Chem. 21, 314 (2019)
G. Li, H. Yang, H. Zhang, Z. Qi, M. Chen, W. Hu, L. Tian, R. Nie, W. Huang, ACS Catal. 8, 8396 (2018)
X. Duan, J. Liu, J. Hao, L. Wu, B. He, Y. Qiu, J. Zhang, Z. He, J. Xi, S. Wang, Carbon 130, 806 (2018)
D. Wang, J. Liu, J. Xi, J. Jiang, Z. Bai, Appl. Surf. Sci 489, 477 (2019)
J. Xi, H. Sun, D. Wang, Z. Zhang, X. Duan, J. Xiao, F. Xiao, L. Liu, S. Wang, Appl. Catal. B-Environ. 225, 291 (2018)
A. Tokarev, J. Yatvin, O. Trotsenko, J. Locklin, S. Minko, Adv. Funct. Mater. 26, 3761 (2016)
S.Z. Li, W. Zhang, M.H. So, C.M. Che, R.M. Wang, R. Chen, J. Mol. Catal. A-Chem. 359, 81 (2012)
W.T. Hu, B.C. Liu, Q. Wang, Y. Liu, Y.X. Liu, P. Jing, S.L. Yu, L.X. Liu, J. Zhang, Chem. Commun. 49, 7596 (2013)
Z.P. Wen, Y.L. Zhang, Y. Wang, L.N. Li, R. Chen, Chem. Eng. J. 312, 39 (2017)
A. Abo Markeb, A. Alonso, A.D. Dorado, A. Sànchez, X. Font, Environ. Technol. 37, 2099 (2016)
M. Seifan, A. Ebrahiminezhad, Y. Ghasemi, A.K. Samani, A. Berenjian, Appl. Microbiol. Biotechnol. 102, 175 (2018)
P. Biehl, M. Von der Lühe, S. Dutz, F.H. Schacher, Polymer 10, 91 (2018)
W. Fu, H. Yang, S. Liu, M. Li, G. Zou, Mater. Lett. 60, 1728 (2006)
S. Taheri, H. Veisi, M. Hekmati, New J. Chem. 41, 5075 (2017)
K.S. Pandit, P.V. Chavan, U.V. Desai, M.A. Kulkarni, P.P. Wadgaonkar, New J. Chem. 39, 4452 (2015)
W.A. Bubb, H.A. Berthon, P.W. Kuchel, Bioorg. Chem. 23, 119 (1995)
T.S. Jin, A.Q. Wang, X. Wang, J.S. Zhang, T.S. Li, Synlett 05, 0871 (2004)
A. Jamshidi, B. Maleki, F.M. Zonoz, R. Tayebee, Mater. Chem. Phys. 209, 46 (2018)
B. Maleki, M. Baghayeri, S.A.J. Abadi, R. Tayebee, A. Khojastehnezhad, RSC Adv. 6, 96644 (2016)
S.F. Hojati, N. MoeiniEghbali, S. Mohamadi, T. Ghorbani, Org. Prep. Proced. Int. 50, 408 (2018)
H. Sharma, S. Srivastava, RSC Adv. 8, 38974 (2018)
M. Hajjami, F. Gholamian, R.H. Hudson, A.M. Sanati, Catal. Lett. 149, 228 (2019)
H. Alinezhad, M. Tarahomi, B. Maleki, A. Amiri, Appl. Organomet. Chem. 33, e4661 (2019)
K.K. Krishnan, V.V. Dabholkar, A. Gopinathan, R. Jaiswar, J. Chem. Chem. Sci. 8, 66 (2018)
M. Norouzi, D. Elhamifar, R. Mirbagheri, Z. Ramazani, J. Taiwan Inst. Chem. Eng. 89, 234 (2018)
M. Norouzi, D. Elhamifar, Catal. Lett. 149, 619 (2019)
M.A. Shaikh, M. Farooqui, S. Abed, Res. Chem. Intermed. 45, 1595 (2019)
M. Gholamhosseini-Nazari, S. Esmati, K.D. Safa, A. Khataee, R. Teimuri-Mofrad, Res. Chem. Intermed. 45, 1841 (2019)
M. Esmaeilpour, J. Javidi, F. Dehghani, F.N. Dodeji, RSC Adv. 5, 26625 (2015)
B. Shitole, N. Shitole, M. Shingare, G. Kakde, Curr. Chem. Lett. 5, 137 (2016)
D.S. Patel, J.R. Avalani, D.K. Raval, J. Saudi Chem. Soc. 20, S401 (2016)
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, M. Norouzi, M. Moein, Res. Chem. Intermed. 41, 8665 (2015)
D. Elhamifar, Z. Ramazani, M. Norouzi, R. Mirbagheri, J. Colloid Interface Sci. 511, 392 (2018)
J. Yang, S. Liu, H. Hu, S. Ren, A. Ying, Chin. J. Chem. Eng. 23, 1416 (2015)
H. Hu, F. Qiu, A. Ying, J. Yang, H. Meng, Int. J. Mol. Sci. 15, 6897 (2014)
J. Davarpanah, A.R. Kiasat, S. Noorizadeh, M. Ghahremani, J. Mol. Catal. A-Chem. 376, 78 (2013)
F. Adibian, A.R. Pourali, B. Maleki, M. Baghayeri, A. Amiri, Polyhedron 175, 114179 (2019)
F. Ataie, A. Davoodnia, A. Khojastehnezhad, Polycycl. Aromat. Compd. 1 (2019)
E. Mollashahi, M. Nikraftar, J. Saudi Chem. Soc 22, 42 (2018)
B. Maleki, Org. Prep. Proced. Int. 48, 3 (2016)
Acknowledgements
The authors gratefully appreciate the partial support from the Research Council of University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faroughi Niya, H., Hazeri, N., Rezaie Kahkhaie, M. et al. Preparation and characterization of MNPs–PhSO3H as a heterogeneous catalyst for the synthesis of benzo[b]pyran and pyrano[3,2-c]chromenes. Res Chem Intermed 46, 1685–1704 (2020). https://doi.org/10.1007/s11164-019-04056-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04056-z