Abstract
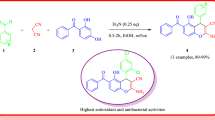
In this study, new derivatives of aminoanthraquinone have been synthesized via one-pot three-component condensation reaction of 1- and 2-amino anthraquinones, triethyl orthoformate and CH-acid compounds without using any solvent or catalyst in mild temperature (50 °C). This simple and efficient method yields the desired products in short reaction time (14–50 min) and good to excellent yields (85–96%). Moreover, chemical structures of synthesized products have been entirely confirmed by FT-IR, 1H and 13CNMR and mass spectroscopy and melting points.



Similar content being viewed by others
References
V.K. Gupta, A. Nayak, S. Agarwal, Environ. Eng. Res. 20, 1 (2015)
R. Saravanan, E. Thirumal, V.K. Gupta, V. Narayanan, A. Stephen, J. Mol. Liq. 177, 394 (2013)
M. Ahmaruzzaman, V.K. Gupta, Ind. Eng. Chem. Res. 50, 13589 (2011)
R. Saravanan, R. Mansoob Khan, V.K. Gupta, E. Mosquera, F. Gracia, V. Narayanan, A. Stephen, J. Colloid Interface Sci. 452, 126 (2015)
R. Saravanan, M.M. Khan, V.K. Gupta, E. Mosquera, F. Gracia, V. Narayanan, A. Stephen, RSC Adv. 5, 34645 (2015)
M. Ghaedi, S. Hajjati, Z. Mahmudi, I. Tyagi, S. Agarwal, A. Maity, V.K. Gupta, Chem. Eng. J. 268, 28 (2015)
R. Saravanan, N. Karthikeyan, V.K. Gupta, E. Thirumal, P. Thangadurai, V. Narayanan, A. Stephen, Mater. Sci. Eng. 33, 2235 (2013)
V.K. Gupta, S.I. Tyagi, S. Agarwal, R. Singh, M. Chaudhary, A. Harit, S. Kushwaha, Global J. Environ. Sci. Manage 2, 1 (2016)
R. Saravanan, V.K. Gupta, V. Narayanan, A. Stephen, J. Taiwan Inst. Chem. Eng. 45, 1910 (2014)
P. Anastas, N. Eghbali, Chem. Soc. Rev. 39, 301 (2010)
P.T. Anastas, M.M. Kirchhoff, Acc. Chem. Res. 35, 686 (2002)
M. Poliakoff, P. Licence, Nature 450, 810 (2007)
F.M. Kerton, R. Marriott, Alternative Solvents for Green Chemistry, 2nd edn. (RSC Publishina, RSC Green Chemistry Series, 2013).
A. Davoodnia, A. Khojastehnezhad, J. Chil. Chem. Soc. 57, 1385 (2012)
A. Khojastehnezhad, F. Moeinpour, M. Vafaei, J. Mex. Chem. Soc. 59, 29 (2015)
C. Capello, U. Fischer, K. Hungerbühler, Green Chem. 9, 927 (2007)
P.J. Dunn, Chem. Soc. Rev. 41, 1452 (2012)
F. Moeinpour, A. Khojastehnezhad, J. Chem. 9, 504 (2012)
M. Aghaei, A.H. Kianfar, M. Dinari, J. Iran. Chem. Soc. 16, 2489 (2019)
C.J. Li, Chem. Rev. 105, 3095 (2005)
C.J. Li, L. Chen, Chem. Soc. Rev. 35, 68 (2006)
F. Tajfirooz, A. Davoodnia, M. Pordel, M. Ebrahimi, A. Khojastehnezhad, Appl. Organomet. Chem. 32, 3930 (2018)
S. Patil, A. Mane, S. Dhongade-Desai, J. Iran. Chem. Soc. 16, 1665 (2019)
T. Palma, B. Vieira, J. Nunes, J. Lourenço, O. Monteiro, M.C. Costa, J. Iran. Chem. Soc. 17, 2013 (2020)
A. Javid, A. Khojastehnezhad, H. Eshghi, F. Moeinpour, F.F. Bamoharram, J. Ebrahimi, Org. Prep. Proc. Int. 48, 377 (2016)
F. Moeinpour, A. Khojastehnezhad, Arab. J. Chem. 10, 3468 (2017)
A. Khojastehnezhad, F. Moeinpour, A. Javid, Polycyc. Aromat. Compd. 39, 404 (2017)
H. Beyzaei, Z. Khosravi, R. Aryan, B. Ghasemi, J. Iran. Chem. Soc. 16, 2565 (2019)
M. Hamidinasab, A. Mobinikhaledi, J. Iran. Chem. Soc. 16, 1255 (2019)
I. Devi, P.J. Bhuyan, Tetrahedron Lett. 45, 8625 (2004)
M.R. Mousavi, M.T. Maghsoodlou, N. Hazeri, S.M. Habibi-Khorassani, J. Iran. Chem. Soc. 12, 1419 (2015)
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, S. Mohamadian-Souri, Res. Chem. Intermed. 42, 2805 (2016)
Z. Ghadamyari, A. Shiri, A. Khojastehnezhad, S.M. Seyedi, Appl. Organomet. Chem. 33, 5091 (2019)
M. Keyhaniyan, A. Shiri, H. Eshghi, A. Khojastehnezhad, Appl. Organomet. Chem. 32, 4344 (2018)
E. Ruijter, R. Scheffelaar, R.V. Orru, Angew. Chem. Int. 50, 6234 (2011)
M. Zhang, H. Jiang, H. Liu, Q. Zhu, Org. Lett. 9, 4111 (2007)
M. Rohaniyan, A. Davoodnia, S.A. Beyramabadi, A. Khojastehnezhad, Appl. Organomet. Chem. 33, 4881 (2019)
M. Rahimizadeh, S.M. Seyedi, M. Abbasi, H. Eshghi, A. Khojastehnezhad, F. Moeinpour, M. Bakavoli, J. Iran. Chem. Soc. 12, 839 (2015)
S. Allameh, A. Davoodnia, A. Khojastehnezhad, Chin. Chem. Lett. 23, 17 (2020)
G. Diaz-Munoz, I.L. Miranda, S.K. Sartori, D.C. de Rezende, M.A. Diaz, in Stud. Nat. Prod. Chem. 58, 313 (2018)
P.M. Dey, J.B. Harborne, Plant Biochemistry (Academic Press, Elsevier, 1997).
E.M. Malik, C.E. Müller, Med. Res. Rev. 36, 705 (2016)
Y. Igarashi, M.E. Trujillo, E. Martínez-Molina, S. Yanase, S. Miyanaga, T. Obata, H. Sakurai, I. Saiki, T. Fujita, T. Furumai, Bioorg. Med. Chem. Lett. 17, 3702 (2007)
L. Dufossé, Food Res. Int. 65, 132 (2014)
H. Langhals, Angew. Chem. Int. Ed. 43, 5290 (2004)
R.M. Christie, Polym. Int. 34, 351 (1994)
M.A. Ackacha, K. Połeć-Pawlak, M. Jarosz, J. Sep. Sci. 26, 1028 (2003)
A. Giwa, F.J. Giwa, B.J. Ifu, Chem. Sci. Int. J. 2, 60 (2012)
M. Malmir, R. Serrano, O. Silva, Res. Inst. Med., 1649 (2013)
D.L. Barnard, D. Fairbairn, K. O’Neill, T. Gage, R. Sidwell, Antiviral Res. 28, 317 (1995)
J.H. Doughari, P.A. Ndakidemi, I.S. Human, S. Benade, J. Ethnopharmacol. 141, 1041 (2012)
S. Imran, A. Hossain, S. Parui, P.S. Sengupta, S. Roy, P.S. Guin, J. Mol. Liq. 252, 151 (2018)
S.Z. Choi, S.O. Lee, K.U. Jang, S.H. Chung, S.H. Park, H.C. Kang, E.Y. Yang, H.J. Cho, K.R. Lee, Arch. Pharmacal Res. 28, 1027 (2005)
Z. Aelami, M.T. Maghsoodlou, R. Heydari, Chem. Select 4, 5315 (2019)
G. Marandi, M.T. Maghsoodlou, R. Heydari, S.M. Habibi-Khorassani, R. Kabiri, Z. Gharechahi, M. Ghahramaninezhad, B. Adrom, Phosphorus Sulfur Silicon Relat. Elem. 185, 1395 (2010)
Z. Aelami, M.T. Maghsoodlou, R. Heydari, A. Yazdani-Elah-Abadi, Polycyc. Aromat. Compd. (2020) https://doi.org/10.1080/10406638.2020.1747096
N.A. Pianovich, M. Dean, A. Lassak, K. Reiss, B.S. Jursic, Bioorg. Med. Chem. 25, 5068 (2017)
Acknowledgements
We wish to express our thanks to the Research Council of the University of Sistan and Baluchestan for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sardashti-Birjandi, A., Mollashahi, E., Maghsoodlou, M.T. et al. Green and catalyst-free synthesis of aminoanthraquinone derivatives in solvent-free conditions. Res Chem Intermed 47, 3597–3608 (2021). https://doi.org/10.1007/s11164-021-04485-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04485-9