Abstract
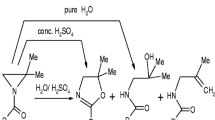
The results of 1H NMR and quantum chemical studies of hydrolysis of isobutylaluminum aryloxides are presented. According to the data of 1H NMR spectroscopy, the hydrolysis of monomeric diisobutylaluminum aryloxides (2,6-Bu2 t—C6H3O)AlBu2 i and (2,6-Bu2 t,4-Me—C6H2O)AlBu2 i occurs selectively at the Al—OAr bond to form the corresponding sterically bulky phenol and polyisobutylaluminoxane. At the molar ratios Al: H2O = 2, the formed sterically bulky phenol reacts slowly with diisobutylaluminum monoaryloxide to form isobutylaluminum diaryloxide. Dimeric aryloxide [(2-But—C6H4O)AlBu2 i]2 is not hydrolyzed under similar conditions. The quantum chemical calculations confirmed the experimental results: the hydrolysis at the Al—OAr bond has a lower energy barrier than that at the Al—C bond because of the formation of \({H_{{H_2}O}} \ldots {O_{O - Ar}}\) hydrogen bonds.
Similar content being viewed by others
References
W. Kaminsky, H. Sinn, Adv. Polym. Sci., 2013, 258, 1.
M. C. Baier, M. A. Zuideveld, S. Mecking, Angew. Chem., Int. Ed., 2014, 53, 2.
K. P. Bryliakov, E. P. Talsi, Coord. Chem. Rev., 2012, 256, 2994.
E. Y. Chen, T. J. Marks, Chem. Rev., 2000, 100, 1391.
A. Forestière, H. Olivier-Bourbigou, L. Saussine, Oil, Gas Sci. Technol. — Rev. IFP, 2009, 64, 649.
Y. V. Kissin, Macromolecules, 2003, 36, 7413.
Y. V. Kissin, Macromol. Rapid Commun., 2004, 25, 1554.
I. Tritto, L. Boggioni, M. C. Sacchi, T. Dall’Occo, J. Mol. Cat. A: Chem., 2003, 204–205, 305.
T. J. Marks, X. Yang, S. B. Mirviss, US Pats 5,391,793 (Feb. 1995), 5,939,346 (Aug. 1999).
Y. V. Kissin, Int. Pat. Appl. WO99/30821 (June 1999).
Y. V. Kissin, Eur. Pat. Appl. EP1062041 (Nov. 1998), US Pat. 6,015,766 (Jan. 2000).
V. Busico, R. Cipullo, F. Cutillo, N. Friederichs, S. Ronca, B. Wang, J. Am. Chem. Soc., 2003, 125, 12402.
D. Romano, E. A. Andablo-Reyes, S. Ronca, S. Rastogi, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1630.
N. H. Tran, S. D. L. Devenport, D. B. Malpass, C. S. Rabbit, US Pat. 5,329,032 (Jul. 1994).
C. N. McMahon, A. R. Barron, J. Chem. Soc., Dalton Trans., 1998, 3703.
R. A. Stapleton, B. R. Galan, S. Collins, R. S. Simons, J. C. Garrison, W. J. Youngs, J. Am. Chem. Soc., 2003, 125, 9246.
R. A. Stapleton, A. Al-Humydi, J. Chai, B. R. Galan, S. Collins, Organometallics, 2006, 25, 5083.
N. M. Bravaya, E. E. Faingol’d, O. N. Babkina, S. L. Saratovskikh, A. N. Panin, I. V. Zharkov, E. A. Fushman, Russ. Chem. Bull. (Int. Ed.), 2013, 62, 560 [Izv. Akad. Nauk, Ser. Khim., 2013, 558].
N. M. Bravaya, A. N. Panin, E. E. Faingol’d, S. L. Saratovskikh, O. N. Babkina, I. V. Zharkov, E. O. Perepelitsina, Polym. Bull., 2016, 73, 473.
Е. Е. Faingol’d, N. M. Bravaya, A. N. Panin, O. N. Babkina, S. L. Saratovskikh, V. I. Privalov, J. Appl. Polym. Sci., 2016, 133, 43276.
A. Bergner, M. Dolg, W. Kuechle, H. Stoll, H. Preuss, Mol. Phys., 1993, 80, 1431.
J. Tomasi, B. Mennucci, R. Cammi, Chem. Rev., 2005, 105, 2999.
R. Benn, E. Janssen, H. Lehmkuhl, A. Rufiñska, K. Angermund, P. Betz, R. Goddard, K. Krüger, J. Organomet. Chem., 1991, 411, 37.
M. D. Healy, A. R. Barron, Angew. Chem., Int. Ed., 1992, 31, 921.
M. D. Healy, D. A. Wierda, A. R. Barron, Organometallics, 1988, 7, 2543.
G. A. Razuvaev, Yu. A. Sangalov, Yu. Ya. Nel’kenbaum, K. S. Minsker, Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1975, 24, 2434 [Izv. Akad. Nauk SSSR, Ser. Khim., 1975, 2547].
T.-L. Yu, C.-H. Huang, L.-F. Yang, B.-T. Ko, C.-C. Lin, J. Chin. Chem. Soc., 2000, 47, 1185.
Y. R. Luo, Comprehensive Handbook of Chemical Bond Energies, CRC Press, Boca, Raton, 2007.
M. D. Healy, J. W. Ziller, A. R. Barron, Organometallics, 1991, 10, 597.
M. D. Healy, J. T. Leman, A. R. Barron, J. Am. Chem. Soc., 1991, 113, 2776.
Y. Yao, G. Qi, Q. Shen, J. Hu, Y. Lin, Chin. Sci. Bull., 2003, 48, 2164.
C. J. Harlan, M. R. Mason, A. R. Barron, Organometallics, 1994, 13, 2957.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faingol’d, E.E., Zharkov, I.V., Bravaya, N.M. et al. Hydrolysis of isobutylaluminum aryloxides studied by 1H NMR and quantum chemical methods. Russ Chem Bull 65, 1958–1965 (2016). https://doi.org/10.1007/s11172-016-1536-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-016-1536-3