Abstract
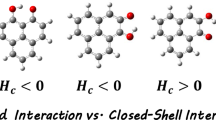
A comprehensive theoretical investigation of the mutual effects of the cation-π, anion-π, and intramolecular hydrogen bond (IMHB) interactions in the various ternary complexes of 1,3,5-triamino-2,4,6-trinitrobenzene (ANB) with anions (A: F˗, Cl˗, Br˗) and cations (M: Li+, Na+, K+, Mg2+, Ca2+) were carried out. The energetic, geometrical, topological, and molecular orbital descriptors are employed to estimate the strength of the mentioned non-covalent interactions and their results were compared with the corresponding data of the ANB⋯M, ANB⋯A and A⋯Bz⋯M complexes, as a set of reference points. The results indicate that the presence of resonance-assisted hydrogen bond (RAHB) units decreases the strength of cation-π interaction while for anion-π interactions, the reverse process is observed. On the other hand, the RAHB units reduce the binding energies of the ternary complexes and cooperative effects between the cation-π and anion-π interactions, with respect to the A⋯Bz⋯M complexes. Furthermore, the coexistence of cation-π and anion-π interactions modulates their effects on the strength of IMHB. Finally, the harmonic oscillator model of aromaticity (HOMA) indicator was applied to analyze the influences of the mentioned interactions on the significance of the π-electron delocalization (π-ED) of the RAHB units and aromaticity of the benzene ring. It was found that the coexistence of cation-π and anion-π interactions effectively change the π˗ED and aromaticity.





Similar content being viewed by others
References
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York,
Scheiner S (1997) Hydrogen bonding. A theoretical perspective. Oxford University Press, New York,
Gilli G, Gilli P (2009) The nature of hydrogen bond. Oxford University Press, New York,
Gilli G, Gilli P (2000) J Mol Struct 552:1–5
Gilli P, Gilli G (2010) J Mol Struct 972:2–10
Bertolasi V, Gilli P, Ferretti V, Gilli G (1991) J Am Chem Soc 113:4917–4925
Gilli G, Bellucci F, Ferretti V, Bertolasi V (1989) J Am Chem Soc 111:1023–1028
Gilli P, Bertolasi V, Ferretti V, Gilli G (1994) J Am Chem Soc 116:909–915
Gilli P, Bertolasi V, Pretto L, Lyčka A, Gilli G (2002) J Am Chem Soc 45:13554–13567
Grabowski SJ (ed.) (2006) Hydrogen bonding: new insights. Springer, Dordrecht,
Grabowski SJ (2001) J Mol Struct (THEOCHEM) 562:137–143
Palusiak M, Simon S, Sola M (2009) J Org Chem 74:2059–2066
Pakiari AH, Eskandari K (2006) J Mol Struct (THEOCHEM) 759:51–60
Woodford JN (2007) J Phys Chem A 111:8519–8530
Hajiabadi H, Nowroozi A, Hasani M, Mohammadzadeh Jahani P, Raissi H (2012) Int J Quantum Chem 112:1384–1391
Nowroozi A, Sarhadinia S, Masumian E, Nakhaei E (2014) Struct Chem 25:1359–1368
Jablonski M, Kaczmarek A, Sadlej AJ (2006) J Phys Chem A 110:10890–10898
Hargis JC, Evangelista FA, Ingels JB, Schaefer HF (2008) J Am Chem Soc 130:17471–1747811
Nowroozi A, Raissi H (2006) J Mol Struct (THEOCHEM) 759:93–100
Nowroozi A, Rezvani Rad O (2017) Theor Chem Account 136:23
Rezvani Rad O, Nowroozi A (2017) Mol Phys 115:784–794
Rezvani Rad O, Nowroozi A (2017) Struct Chem 1–9. doi:10.1007/s11224-017-0921-3
Hobza P, Moller-Dethlefs K (2010) Non-covalent interactions: theory and experiment. Royal Society of Chemistry, Cambridge,
Scheiner S (ed) Noncovalent forces. Springer (2015)
Frontera A, Quinonero D, Deya PM (2011) Wiley Interdisciplinary Reviews Computational Molecular Science 1:440–459
Ebrahimi A, Habibi M, Sayyadi O (2008) J Mol Struct: THEOCHEM 859:46–50
Ebrahimi A, Habibi M, Sayyad O (2008) Mol Simul 34:689–697
Escudero D, Frontera A, Quiñonero D, Deyá PM (2008) Chem Phys Lett 456:257–261
Estarellas C, Frontera A, Quiñonero D, Deyá PM (2009) Chem Phys Lett 479:316–320
Estarellas C, Escudero D, Frontera A, Quiñonero D, Deyá PM (2009) Theor Chem Account 122:325–332
Ebrahimi A, Masoodi HR, Habibi Khorassani A, Hoseini Ghaleno M (2012) Comput Theor Chem 988:48–55
Rooman M, Liévin J, Buisine E, Wintjens R (2002) J Mol Biol 319:67–76
Schottel BL, Chifotides HT, Dunbar KR (2008) Chem Soc Rev 37:68–83
Krygowski TM, Cyranski MK (1996) Tetrahedron 52:1713–1722
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zarzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, KomaromiI GR, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2003) Gaussian 03 program package. Gaussian, Inc., Pittsburgh,
Boys SF, Bernardi F (1970) Mol Phys 19:553–556
Simon S, Duran M, Dannenberg JJ (1996) J Chem Phys 105:11024–11031
Biegler KF, Schonbohm J, Bayles D (2001) AIM2000: a program to analyze andvisualize atoms in molecules J Comp Chem 22:545–559
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1990) NBO, Version 3.1
Nowroozi A, Hajiabadi H, Akbari F (2014) Struc Chem 25:251–258
Espinosa E, Molins E (2000) J Chem Phys 113:5686–5694
Dziembowska T, Szczodrowska B, Krygowski TM, Grabowski SJ (1994) J Phys Org Chem 7:142–146
Wang DX, Wang MX (2013) J Am Chem Soc 135:892–897
Quiñonero D, Garau C, Frontera A, Ballester P, Costa A, Deyà PM (2002) Chem Phys Lett 359:486–492
Estarellas C, Quiñonero D, Frontera A, Ballester P, Morey J, Costa A, Deyà PM (2008) J Phy Chem A 112:1622–1626
Garau C, Frontera A, Quiñonero D, Ballester P, Costa A, Deyà PM (2004) J Phy Chem A 108:9423–9427
Palusiak M, Simon S, Sola M (2006) J Org Chem 71:5241–5248
Krygowski TM, Bankiewicz B, Czarnocki Z, Palusiak M (2015) Tetrahedron 71:4895–4908
Güell M, Poater J, Luis JM, Mó O, Yáñez M, Solà M (2005) Chem Phys Chem 6:2552–2561
Nowroozi A, Nakhaei E, Masumian E (2014) Struc Chem 25:1415–1422
Nakhaei E, Nowroozi A (2016) Computational and Theoretical Chemistry 1096:27–32
Acknowledgments
The authors thank the University of Sistan and Baluchestan (USB) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional file
ESM 1
Fig. 1S Molecular structures of a ANB⋯A, ANB⋯M and b A⋯Bz⋯M complexes (M = Li+, Na+, K+, Mg2+ and Ca2+, A = F−, Cl−, and Br−). Fig. 2S Some typical molecular graphs obtained from AIM analysis for a F−⋯Bz⋯Ca2+, Cl−⋯Bz⋯Mg2+, b F−⋯Bz⋯Li+, F−⋯Bz⋯Na+, F−⋯Bz⋯K+ c Cl−⋯Bz⋯Ca2+, d other A⋯Bz⋯M, e ANB⋯A and ANB⋯M complexes. Small red spheres represent bond critical points (BCPs). Fig. 3S Correlations of the dN…O swith the EA…ANB…M, EHB and ECOOP values. Fig. 4S The ρπ…M and ρπ…A parameters versus the EHB values. Fig. 5S The ρH…O parameter versus the EA…ANB…M and ECOOP values. Table 1S The binding energies (kcal mol−1) at M062X/6–311++G(d,p) level of theory. Table 2S The geometrical descriptors (Ǻ) of A⋯Bz⋯M complexes. Table 3S The selected topological properties of electron density (a.u. × 102) obtained by AIM analysis. Table 2S The selected results of NBO analysis at M062X/6–311++G(d,p) level of theory. Table 2S The HOMA values of A⋯Bz⋯M complexes. (DOCX 408 kb)
Rights and permissions
About this article
Cite this article
Nowroozi, A., Ebrahimi, A. & Rezvani Rad, O. Mutual effects of the cation-π, anion-π and intramolecular hydrogen bond in the various complexes of 1,3,5-triamino-2,4,6-trinitrobenzene with some cations (Li+, Na+, K+, Mg2+, Ca2+) and anions (F˗, Cl˗, Br˗). Struct Chem 29, 129–137 (2018). https://doi.org/10.1007/s11224-017-1010-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-017-1010-3