Abstract
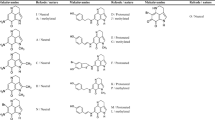
Among physiologically active compounds isolated from marine organisms, the pyrroloquinoline alkaloids (PQAs) family consisting of damirone A/C, batzelline A/D, makaluvamine O and makaluvone exhibit numerous biological activities derived from the inimitable highly-fused structures. Their structural and electronic properties were investigated through intramolecular interactions, electron affinities (EA), reduction potentials (E°) and complexation of compounds with magnesium and guanidinium cations (ionic and hydrogen bond interactions) using quantum mechanical calculations in the gas phase and solution. For both series of complexes, the most and least stable ones correspond to damirone A and batzelline D, respectively. The energy data, geometrical parameters, and the minima of electrostatic potentials (Vmin) are in good correlation with the results of population analyses. The localized molecular orbital energy decomposition analyses (LMO-EDA) demonstrate that the electrostatic interaction is the most important stabilizing component. The EA values are positive in the gas phase and solution, and are evaluated with the increase in the dielectric constant of solvent. Increase in the EA values and decrease in the E° values are observed after complexation with guanidinium and magnesium cations. Those changes are higher for electron-rich compounds compared to electron-deficient ones, containing halogen substituents.









Similar content being viewed by others
References
Heffner JE, Raber JC, Moe Jr OA, Wigal CT (1998). J Chem Educ 75:365
Zhang M, Vervoort L, Moalin M, Mommers A, Douny C, den Hartog GJ, Haenen GR (2018). Free Radic Biol Med 124:31–39
Y Zhu Y, Wu M, Gao N, Chu W, Zhao L, Wang Q (2019). Chem Eng J 357:75–83
Bolton JL, Dunlap TL, Dietz BM (2018). Food Chem Toxicol 120:700–707
Reynolds CA (1990). J Am Chem Soc 112:7545–7551
Ferguson-Miller S, Babcock GT (1996). Chem Rev 96:2889–2908
Fukuzumi S, Guldi D M (2001) Electron transfer in chemistry, V. Balzani (Ed.), Wiley-VCH, Weinheim 2: 270–337
Lewis FD (2001) Electron transfer in chemistry, V. Balzani (Ed.), Wiley-VCH, Weinheim 3: 105–175
Fukuzumi S (2003). Org Biomol Chem 1:609–620
Turek AK, Hardee DJ, Ullman AM, Nocera DG, Jacobsen EN (2016). Angew Chem Int Ed 55:539–544
Yuasa J, Yamada S, Fukuzumi S (2007). Angew Chem Int Ed 46:3553–3555
Macıas-Ruvalcaba NA, González I, Aguilar-Martınez M (2004). J Electrochem Soc 151:E110–E118
Fukuzumi S, Kitaguchi H, Suenobu OS (2002) Activation of electron transfer reduction of p-benzoquinone derivatives by intermolecular regioselective hydrogen bond formation. Chem Commun (17):1984–1985
Yuasa J, Yamada S, Fukuzumi S (2008). J Am Chem Soc 130:5808–5820
Okamoto K, Ohkubo K, Kadish KM, Fukuzumi S (2004). J Phys Chem A 108:10405–10413
Greaves MD, Niemz A, Rotello VM (1999). J Am Chem Soc 121:266–267
Gómez M, Gómez-Castro CZ, Padilla-Martı́nez II, Martı́nez-Martı́nez FJ, González FJ (2004). J Electroanal Chem 567:269–276
Namazian M, Coote ML (2007). J Phys Chem A 111:7227–7232
Alvarez M, Bros MA, Gras G, Ajana W, Joule JA (1999). Eur J Org Chem 1999:1173–1183
Backenköhler J, Reck B, Plaumann M, Spiteller P (2018). Eur J Org Chem 2018:2806–2816
Chen QB, Xin XL, Yang Y, Lee SS, Aisa HA (2014). J Nat Prod 77:807–812
Radisky DC, Radisky ES, Barrows LR, Copp BR, Kramer RA, Ireland CM (1993). J Am Chem Soc 115:1632–1638
Carney JR, Scheuer PJ, Kelly-Borges M (1993). Tetrahedron 49:8483–8486
Lohmann JS, Wagner S, von Nussbaum M, Pulte A, Steglich W, Spiteller P (2018). Chem Eur J 24:8609–8614
El-Demerdash A, Atanasov AG, Bishayee A, Abdel-Mogib M, Hooper JN, Al-Mourabit A (2018). Nutrients 10:33
Miyanaga A, Janso JE, McDonald L, He M, Liu H, Barbieri L, Feng X (2011). J Am Chem Soc 133:13311–13313
Jordan PA, Moore BS (2016). Cell Chem Biol 23:1504–1514
Oshiyama T, Satoh T, Okano K, Tokuyama H (2012). Tetrahedron 68:9376–9383
Backenköhler J, Spindler S, Spiteller P (2017). ChemistrySelect 2:2589–2592
Blanco F, Kelly B, Sánchez-Sanz G, Trujillo C, Alkorta I, Elguero J, Rozas I (2013). J Phys Chem B 117:11608–11616
Buchdunger E, Zimmermann J, Mett H, Meyer T, Mueller M, Druker BJ, Lydon NB (1996). Cancer Res 56:100–104
Wexselblatt E, Esko JD, Tor Y (2014). J Organomet Chem 79:6766–6774
Werner J, Wernersson E, Ekholm V, Ottosson N, Öhrwall G, Heyda J, BjÖrneholm O (2014). J Phys Chem B 118:7119–7127
Yuasa J, Suenobu T, Fukuzumi S (2006). Chemphyschem 7:942–954
Orsetti S, Laskov C, Haderlein SB (2013). Environ Sci Technol 47:14161–14168
Casini A, Finazzi-Agrò A, Sabatini S, Tortorella S, Scipione L (1999). Arch Biochem Biophys 368:385–393
Park H, Oyama M (2002) Electroanalysis 14:1269–1274
Witwicki M, Jezierska J (2011). J Phys Chem B 115:3172–3184
Nicolás I, Vilchis M, Aragón N, Miranda R, Hojer G, Castro M (2003). Int J Quantum Chem 93:411–421
Cesaretti A, Carlotti B, Gentili PL, Clementi C, Germani R, Elisei F (2014). J Phys Chem B 118:8601–8613
Deshayes S, Xian W, Schmidt NW, Kordbacheh S, Lieng J, Wang J, Wong GC (2017). Bioconjug Chem 28:793–804
Reed AE, Curtiss LA, Weinhold F (1988). Chem Rev 88:899–926
Su P, Li H (2009). J Chem Phys 131:014102
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.02. Gaussian Inc., Wallingford
Improta R, Barone V (2004). J Comput Chem 25:1333–1341
Moosavi-Tekyeh Z, Taherian F, Tayyari SF (2016). J Mol Struct 1111:185–192
Boys SF, Bernardi FD (1970). Mol Phys 19:553–566
Su P, Jiang Z, Chen Z, Wu W (2014). J Phys Chem A 118:2531–2542
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993). J Comput Chem 14:1347–1363
Bader RFW (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323
Biegler-Konig JSF, Bayles D (2001). J Comput Chem 22:545–559
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Foster JP, Weinhold F (1980). J Am Chem Soc 102:7211
Cai ZL, Sendt K, Reimers JR (2002). J Chem Phys 117:5543–5549
Tomasi J, Mennucci B, Cammi R (2005). Chem Rev 105:2999–3094
Rozas I, Alkorta I, Elguero J (2000). J Am Chem Soc 122:11154–11161
Zhu XQ, Wang CH (2010). J Organomet Chem 75:5037–5047
Bu Y, Liu Y, Deng C (1998). J Mol Struct THEOCHEM 422:219–228
Chen M, Moir D, Benoit FM, Kubwabo C (1998). J Chromatogr A 825:37–44
Nepal B, Scheiner S (2016). J Organomet Chem 81:4316–4324
Bím D, Rulíšek L, Srnec M (2015). J Phys Chem Lett 7:7–13
Fu Y, Liu L, Wang YM, Li JN, Yu TQ, Guo QX (2006). J Phys Chem A 110:5874–5886
Cheng GJ, Song LJ, Yang YF, Zhang X, Wiest O, Wu YD (2013). ChemPlusChem 78:943–951
Baik MH, Friesner RA (2002). J Phys Chem A 106:7407–7412
Jacobs N, Lang S, Panisch R, Wittstock G, Groth U, Nasiri HR (2015). RSC Adv 5:58561–58565
Raymond KS, Grafton AK, Wheeler RA (1997). J Phys Chem B 101:623–631
Hesabi N, Ebrahimi A, Nowroozi A (2017). J Mol Graph Model 77:86–93
Acknowledgments
We are grateful to the University of Sistan and Baluchestan for financial support and the Computational Quantum Chemistry Laboratory for computational facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 155 kb)
Rights and permissions
About this article
Cite this article
Chalanchi, S.M., Ebrahimi, A. & Nowroozi, A. Complexes of damirone A/C, batzelline A/D, makaluvamine O and makaluvone with guanidinium and magnesium cations: a theoretical study. Struct Chem 30, 1635–1646 (2019). https://doi.org/10.1007/s11224-019-01325-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01325-w