Abstract
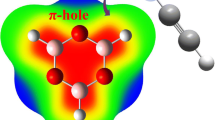
Ab initio calculations have been performed to study the complexes between the noble gas elements (Ae = Ne, Ar, Kr) with a series of benzene isoelectronic heterocyclic compounds of benzene, including boraphosphinine (BP), borazine (BN), and alumazine (AlN), at MP2/Aug-CC-pVTZ level of theory. According to the molecular electrostatic potential (MEP) iso-surface of BP, BN, and AlN, the active sites of rings are identified which utilized to predict the relative strength of aerogen···π (Ae···π) interactions as follows: Kr···π > Ar···π > Ne···π/Ae···BP > Ae···BN > Ae···AlN. Then, the equilibrium structures of all the complexes are characterized, and their energetic, geometrical, topological, and molecular orbital descriptors were used to estimate the strength of Ae···π interactions, that are in line with MEP results. Energy decomposition analysis reveals that dispersion effects play a vital role in formation of the Ae···π complexes. Furthermore, intermolecular interactions were also investigated with the quantum theory of atoms in molecules (QTAIM) and the non-covalent interactions (NCI) and natural bond orbital’s (NBO) analysis. NBO analysis showed that like the benzene complexes, charge transfers from the noble gas atom to heterocyclic ring have occurred. Finally, the aromaticity of the rings is measured using the well-established indices namely the nucleus independent chemical shift (NICS) and the average two-center index (ATI).





Similar content being viewed by others
References
Janiak C (2000) A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J Chem Soc Dalton Trans 21:3885–3896
Bavafa S, Nowroozi A, Ebrahimi A (2019) Quantum chemical study of the nature of interactions between the boraphosphinine and alumaphosphinine with some of the mono- and divalent cations: cation–π or cation–lone pair? Struct Chem. https://doi.org/10.1007/s11224-019-01320-1
Dougherty DA (2012) The cation− π interaction. Acc Chem Res 46:885–893
Ma JC, Dougherty DA (1997) The cation− π interaction. Chem Rev 97:1303–1324
Ballester P (2012) Experimental quantification of anion− π interactions in solution using neutral host–guest model systems. Acc Chem Res 46:874–884
Schottel BL, Chifotides HT, Dunbar KR (2008) Anion-π interactions. Chem Soc Rev 37:68–83
Mooibroek TJ, Gamez P, Reedijk J (2008) Lone pair–π interactions: a new supramolecular bond? CrystEngComm 10:1501–1515
Egli M, Sarkhel S (2007) Lone pair− aromatic interactions: to stabilize or not to stabilize. Acc Chem Res 40:197–205
Mani D, Arunan E (2014) The X–C··· π (X = F, Cl, Br, Cn) Carbon Bond. J Phys Chem A 118:10081–10089
Nziko VPN, Scheiner S (2015) S··· π Chalcogen bonds between SF2 or SF4 and C–C multiple bonds. J Phys Chem A 119:5889–5897
Estarellas C, Frontera A, Quinonero D, Alkorta I, Deya PM, Elguero J (2009) Energetic vs synergetic stability: a theoretical study. J Phys Chem A 113:3266–3273
Bauzá Riera A, Quiñonero Santiago D, Frontera BA (2018) Pnicogen-pi complexes: theoretical study and biological implications. Phys Chem Chem Phys, 2012 14(40):14061–14066
Salonen LM, Ellermann M, Diederich F (2011) Aromatic rings in chemical and biological recognition: energetics and structures. Angew Chem Int Ed 50:4808–4842
Mahadevi AS, Sastry GN (2012) Cation− π interaction: Its role and relevance in chemistry, biology, and material science. Chem Rev 113:2100–2138
Subramanian S, Zaworotko MJ (1994) Exploitation of the hydrogen bond: recent developments in the context of crystal engineering. Coord Chem Rev 137:357–401
Steed JW, Atwood JL (2009) Supramolecular chemistry. Wiley, Wiltshire
Tan Z, Li AY (2018) Noble gas supported boron-pentagonal clusters B 5 Ng n 3+: exploring the structures and bonding. J Mol Model 24:90
Zierkiewicz W, Michalczyk M, Scheiner S (2018) Aerogen bonds formed between AeOF 2 (Ae = Kr, Xe) and diazines: comparisons between σ-hole and π-hole complexes. Phys Chem Chem Phys 20:4676–4687
Miao J, Gao Y (2017) The switch of the binding behaviours between Xe and π system induced by the change of oxidation state of Cu ion. Mol Simul 43:1256–1259
Gao M, Cheng J, Li W, Xiao B, Li Q (2016) The aerogen–π bonds involving π systems. Chem Phys Lett 651:50–55
Esrafili MD, Mousavian P, Mohammadian-Sabet F (2019) Tuning of pnicogen and chalcogen bonds by an aerogen-bonding interaction: a comparative ab initio study. Mol Phys 117:58–66
Bauzá A, Frontera A (2015) Theoretical study on the dual behavior of XeO3 and XeF4 toward aromatic rings: lone pair–π versus aerogen–π interactions. ChemPhysChem 16:3625–3630
Quiñonero D, Frontera A, Deyà PM (2012) Interplay between ion–π and Ar/π Van der Waals interactions. Comput Theor Chem 998:51–56
Bartlett N (1962) Xenon hexafluoroplatinate (V) Xe + [PtF6]. Royal Soc Chemistry Thomas Graham House, Science Park, Milton Rd, Cambridge, p 218
Claassen HH, Selig H, Malm JG (1962) Xenon tetrafluoride. J Am Chem Soc 84:3593
Hoppe R, Dähne W, Mattauch H, Rödder K (1962) Fluorination of xenon. Angew Chem Int Ed Engl 1:599
Bauzá A, Frontera A (2015) Aerogen bonding interaction: a new supramolecular force? Angew Chem Int Ed 54:7340–7343
Murray JS, Lane P, Politzer P (2009) Expansion of the σ-hole concept. J Mol Model 15:723–729
Politzer P, Murray JS, Clark T (2013) Halogen bonding and other σ-hole interactions: a perspective. Phys Chem Chem Phys 15:11178–11189
Miao J, Song B, Gao Y (2015) Is aerogen–π interaction capable of initiating the noncovalent chemistry of group 18? Chem Asian J 10:2615–2618
Esrafili MD, Asadollahi S (2016) Strengthening of the halogen-bonding by an aerogen bond interaction: substitution and cooperative effects in O3Z··· NCX··· NCY (Z = Ar, Kr, Xe; X = Cl, Br, I; Y = H, F, OH) complexes. Mol Phys 114:2177–2186
Miao J, Song B, Gao Y (2016) Enhanced aerogen–π interaction by a cation–π force. Chem Eur J 22:2586–2589
Suresh CH, Mohan N, Della TD (2018) A noncovalent binding strategy to capture noble gases, hydrogen and nitrogen. J Comput Chem 39:901–908
Tarakeshwar P, Kim KS, Kraka E, Cremer D (2001) Structure and stability of fluorine-substituted benzene-argon complexes: the decisive role of exchange-repulsion and dispersion interactions. J Chem Phys 115:6018–6029
Schmies M, Patzer A, Fujii M, Dopfer O (2011) Structures and IR/UV spectra of neutral and ionic phenol–Ar n cluster isomers (n ≤ 4): competition between hydrogen bonding and stacking. Phys Chem Chem Phys 13:13926–13941
Cao Q, Andrijchenko N, Ermilov A, Räsänen M, Nemukhin A, Khriachtchev L (2014) Interaction of aromatic compounds with xenon: spectroscopic and computational characterization for the cases of p-cresol and toluene. J Phys Chem A 119:2587–2593
López-Tocón I, Otero J, Becucci M, Pietraperzia G, Castellucci E (1999) The aniline–argon van der Waals complex: ab initio second-order Møller–Plesset study of the potential energy surface in the ground electronic state. Chem Phys 249:113–120
Hobza P, Selzle H, Schlag E (1991) Ab initio calculations on the structure, stabilization, and dipole moment of benzene Ar complex. J Chem Phys 95:391–394
Hobza P, Bludský O, Selzle HL, Schlag EW (1996) Ab initio calculations on the structure, vibrational frequencies, and valence excitation energies of the benzene … Ar and benzene … Ar2 cluster. Chem Phys Lett 250:402–408
Hobza P, Bludský O, Selzle H, Schlag E (1992) Ab initio second-and fourth-order Mo/ller–Plesset study on structure, stabilization energy, and stretching vibration of benzene··· X (X = He, Ne, Ar, Kr, Xe) van der Waals molecules. J Chem Phys 97:335–340
Hobza P, Selzle H, Schlag E (1993) Properties of fluorobenzene··· Ar and p-difluorobenzene Ar complexes: ab initio study. J Chem Phys 99:2809–2811
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven Jr T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, KleneM LX, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C (2003) Pople JA Gaussian 03 revision C 02 (or D 01). Gaussian Inc, Pittsburgh
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Boys SF, Fd B (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Millam J, Eppinnett K, Hovell W, Gilliland R (2003) GaussView. Shawnee Mission. KS, Semichem Inc
Keith TA (2013) AIMAll (Version 13.05. 06). TK Gristmill Software, Overland Park, KS, USA
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746
Glendening E, Reed A, Carpenter J, Weinhold F (2001) NBO. Theoretical Chemistry Institute, University of Wisconsin, Madison, USA
Bultinck P, Ponec R, Van Damme S (2005) Multicenter bond indices as a new measure of aromaticity in polycyclic aromatic hydrocarbons. J Phys Org Chem 18:706–718
Schleyer PR, Maerker C, Dransfeld A, Jiao H, van Eikema Hommes NJ (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318
Schleyer PR, Manoharan M, Wang Z-X, Kiran B, Jiao H et al (2001) Dissected nucleus-independent chemical shift analysis of π-aromaticity and antiaromaticity. Org Lett 3:2465–2468
Hameka H (1958) On the nuclear magnetic shielding in the hydrogen molecule. Mol Phys 1:203–215
Politzer P, Laurence PR, Jayasuriya K (1985) Molecular electrostatic potentials: an effective tool for the elucidation of biochemical phenomena. Environ Health Perspect 61:191–202
Murray JS, Politzer P (2011) The electrostatic potential: an overview. Wiley Interdiscip Rev: Comput Mol Sci 1:153–163
Politzer P, Truhlar DG (eds) (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum Press, New York
Rezvani Rad O, Nowrozi A (2018) Anion˗ π and intramolecular hydrogen bond interactions in the various complexes of 1, 3, 5-triamino-2, 4, 6-trinitrobenzene with H-, F-, Cl-and Br-anions. Phys Chem Res 6:251–262
Ebrahimi A, Razmazma H, Samareh DH (2016) The nature of halogen bonds in [N∙∙∙ X∙∙∙ N] + complexes: a theoretical study. Phys Chem Res 4:1–15
Bania KK, Guha AK, Bhattacharyya PK, Sinha S (2014) Effect of substituent and solvent on cation–π interactions in benzene and borazine: a computational study. Dalton Trans 43:1769–1784
Zhang Y, Xu Z (1995) Atomic radii of noble gas elements in condensed phases. Am Mineral 80:670–675
Frontera A, Gamez P, Mascal M, Mooibroek TJ, Reedijk J (2011) Putting anion–π interactions into perspective. Angew Chem Int Ed 50:9564–9583
Sinnokrot MO, Valeev EF, Sherrill CD (2002) Estimates of the ab initio limit for π−π interactions: The benzene dimer. J Am Chem Soc 124:10887–10893
Soteras I, Orozco M, Luque FJ (2008) Induction effects in metal cation–benzene complexes. Phys Chem Chem Phys 10:2616–2624
Varadwaj A, Varadwaj PR, Marques HM, Yamashita K (2018) A DFT assessment of some physical properties of iodine-centered halogen bonding and other non-covalent interactions in some experimentally reported crystal geometries. Phys Chem Chem Phys 20:15316–15329
Panneer SVK, Ravva MK, Mishra BK, Subramanian V, Sathyamurthy N (2018) Co-operativity in non-covalent interactions in ternary complexes: a comprehensive electronic structure theory based investigation. J Mol Model 24:258
Hayashi S, Tsubomoto Y, Nakanishi W (2018) Behavior of the E–E’Bonds (E, E’ = S and Se) in glutathione disulfide and derivatives elucidated by quantum chemical calculations with the quantum theory of atoms-in-molecules approach. Molecules 23:443
Shahi A, Arunan E (2014) Hydrogen bonding, halogen bonding and lithium bonding: an atoms in molecules and natural bond orbital perspective towards conservation of total bond order, inter-and intra-molecular bonding. Phys Chem Chem Phys 16:22935–22952
Shainyan B, Chipanina N, Aksamentova T, Oznobikhina L, Rosentsveig G, Rosentsveig I (2010) Intramolecular hydrogen bonds in the sulfonamide derivatives of oxamide, dithiooxamide, and biuret. FT-IR and DFT study, AIM and NBO analysis. Tetrahedron 66:8551–8556
Wu Q, Su H, Wang H, Wang H (2018) Ab initio calculations, structure, NBO and NCI analyses of XH⋯ π interactions. Chem Phys Lett 693:202–209
Esrafili MD, Sadr-Mousavi A (2017) Chalcogen bonds tuned by an N–H··· π or C–H··· π interaction: investigation of substituent, cooperativity and solvent effects. Mol Phys 115:1713–1723
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Chakraborty D, Chattaraj PK (2018) Confinement induced thermodynamic and kinetic facilitation of some Diels–Alder reactions inside a CB [7] cavitand. J Comput Chem 39:151–160
Lefebvre C, Rubez G, Khartabil H, Boisson J-C, Contreras-García J, Hénon E (2017) Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys Chem Chem Phys 19:17928–17936
Bühl M, van Wüllen C (1995) Computational evidence for a new C84 isomer. Chem Phys Lett 247:63–68
Peng H, Huang P, Yi P, Xu F, Sun L (2018) Theoretical studies of π-electron delocalization and localization on intramolecular proton transfer in the ground state. J Mol Struct 1154:590–595
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bavafa, S., Nowroozi, A. & Ebrahimi, A. Ab initio study of aerogen-bonds between some heterocyclic compounds of benzene with the noble gas elements (Ne, Ar, and Kr). Struct Chem 31, 435–445 (2020). https://doi.org/10.1007/s11224-019-01416-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01416-8