Abstract
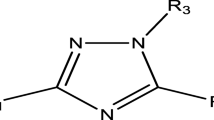
Tuberculosis (TB), an infectious remains a global health burden till date. Considering immense importance of theoretical tools in computer aided-drug designing, the current study focuses on common pharmacophore and QSAR analysis of 38 imidazopyridine analogues as anti-TB agents. Our developed atom-based, field-based, and multilinear regression based-QSAR models showed high values for statistical robustness for internal as well as external validations (a correlation coefficient: R2 > 0.9, least standard deviations, higher Fischer coefficient, and cross-validation correlation coefficient: Q2 > 0.5). From our ZINC-Drug-like analysis, we were retained with 5 hits (VS1-VS-5), among them VS-4 molecule was found to have high potency (predicted pIC50 (μM) value: 7.96 (against MTB H37Rv ATCC 27,294)) with good theoretical properties (high softness, and low hardness values). From our designed analogues (S1-S10), analogue S-10 was retained with high potency as well as good pharmacokinetics to act as good anti-mycobacterial agent in future.










Similar content being viewed by others
Data availability statement
Data will be made available upon reasonable request.
Abbreviations
- TB:
-
Tuberculosis
- QSAR model:
-
Quantitative structure–activity relationship model
- DFT:
-
Density functional theory
- MDR-TB:
-
Multi-drug-resistant tuberculosis
- XDR-TB:
-
Extensively drug-resistant tuberculosis
- 3D-QSAR model:
-
3D-quantitative structure–activity relationship models
- CPH:
-
Common pharmacophore hypothesis
- R 2 :
-
Correlation coefficient
- Q 2 :
-
Cross-validation correlation coefficient
- RMSE:
-
Root mean square error
- ADMET:
-
Absorption, distribution, metabolism, excretion and toxicity properties
- MIC:
-
Minimum inhibitory concentration
- IC50 :
-
Inhibitory concentration
- PLS:
-
Partial least squares
References
Mali SN, Chaudhari HK (2018) Computational studies on imidazo [1, 2-a] pyridine-3-carboxamide analogues as antimycobacterial agents: Common pharmacophore generation, atom-based 3D-QSAR, molecular dynamics simulation, QikProp, molecular docking and prime MMGBSA approaches. Open Pharm Sci J 5(1). https://doi.org/10.2174/1874844901805010012
Tuberculosis - WHO (World Health Organization) Factsheet, Accessed online on: 21-01-2022. https://www.who.int/news-room/fact-sheets/detail/tuberculosis#
Alemu MA, Yesuf A, Girma F, Adugna F, Melak K, Biru M, Seyoum M, Abiye T (2021) Impact of HIV-AIDS on tuberculosis treatment outcome in Southern Ethiopia–a retrospective cohort study. J Clin Tuberc Other Mycobact Dis 25:100279
Lv K, Li L, Wang B et al (2017) Design, synthesis and antimycobacterial activity of novel imidazo[1,2-a]pyridine-3-carboxamide derivatives. Eur J Med Chem 137:117–125
Khatun S, Singh A, Bader GN, Sofi FA (2021) Imidazopyridine, a promising scaffold with potential medicinal applications and structural activity relationship (SAR): recent advances. J Biomol Struct Dyn 1–24
Kshatriya R, Kambale D, Mali S, Jejurkar VP, Lokhande P, Chaudhari HK, Saha SS (2019) Brønsted acid catalyzed domino synthesis of functionalized 4H-chromens and their ADMET, molecular docking and antibacterial studies. ChemistrySelect 4(27):7943–7948. https://doi.org/10.1002/slct.201901775
Jejurkar VP, Mali SN, Kshatriya R, Chaudhari HK, Saha S (2019) Synthesis, antimicrobial screening and in silico appraisal of iminocarbazole derivatives. ChemistrySelect 4(32):9470–9475. https://doi.org/10.1002/slct.201901890
Jadhav BS, Yamgar RS, Kenny RS, Mali SN, Chaudhari HK, Mandewale MC (2020) Synthesis, in silico and biological studies of thiazolyl-2h-chromen-2-one derivatives as potent antitubercular agents. Curr Comput Aided Drug Des 16(5):511–522. https://doi.org/10.2174/1386207322666190722162100
Desale VJ, Mali SN, Chaudhari HK, Thorat BR, Yamgar RS (2020) Synthesis and Anti-mycobacterium Study on Halo-substituted 2-aryl oxyacetohydrazones. Curr Comput Aided Drug Des 16(5):618–628. https://doi.org/10.2174/1573409915666191018120611
Anuse DG, Mali SN, Thorat BR, Yamgar RS, Chaudhari HK (2020) Synthesis, SAR, In silico appraisal and anti-microbial study of substituted 2-aminobenzothiazoles derivatives. Curr Comput Aided Drug Des 16(6):802–813. https://doi.org/10.2174/1573409915666191210125647
Thorat BR, Rani D, Yamgar RS, Mali SN (2020) Synthesis, spectroscopic, in-vitro and computational analysis of hydrazones as potential antituberculosis agents:(part-I). Comb Chem High Throughput Screening 23(5):392–401. https://doi.org/10.2174/1386207323999200325125858
Leão RP, Cruz JV, da Costa GV, Cruz JN, Ferreira EFB, Silva RC, de Lima LR, Borges RS, dos Santos GB, Santos CBR (2020) Identification of new rofecoxib-based cyclooxygenase-2 inhibitors: a bioinformatics approach. Pharmaceuticals 13:209. https://doi.org/10.3390/ph13090209
Santana de Oliveira M, da Cruz JN, Almeida da Costa W, Silva SG, Brito MdP, de Menezes SAF, de Jesus Chaves Neto AM, de Aguiar Andrade EH, de Carvalho Junior RN (2020) Chemical Composition, Antimicrobial Properties of Siparuna guianensis Essential Oil and a Molecular Docking and Dynamics Molecular Study of its Major Chemical Constituent. Molecules 25(17):3852. https://doi.org/10.3390/molecules25173852
Araújo PHF, Ramos RS, da Cruz JN, Silva SG, Ferreira EFB, de Lima LR, Macêdo WJC, Espejo-Román JM, Campos JM, Santos CBR (2020) Identification of potential COX-2 inhibitors for the treatment of inflammatory diseases using molecular modeling approaches. Molecules 25(18):4183. https://doi.org/10.3390/molecules25184183
Neto RDAM, Santos CBR, Henriques SVC, Machado LDO, Cruz JN, da Silva CHTP, Federico LB, de Oliveira EHC, de Souza MPC, da Silva PNB, Taft CA, Ferreira IM, Gomes MRF (2020) Novel chalcones derivatives with potential antineoplastic activity investigated by docking and molecular dynamics simulations. J Biomol Struct Dyn https://doi.org/10.1080/07391102.2020.1839562
Santana de Oliveira M, Pereira da Silva VM, Cantão Freitas L, Gomes Silva S, Nevez Cruz J, de Aguiar Andrade EH (2021) Extraction yield, chemical composition, preliminary toxicity of Bignonia nocturna (Bignoniaceae) essential oil and in silico evaluation of the interaction. Chem Biodivers 18(4):e2000982
Costa EB, Silva RC, Espejo-Román JM, de A. Neto MF, Cruz JN, Leite FHA, Silva CHTP, Pinheiro JC, Macêdo WJC, Santos CBR (2020) Chemometric methods in antimalarial drug design from 1,2,4,5-tetraoxanes analogues, SAR and QSAR in Environmental Research 31(9):677-695. https://doi.org/10.1080/1062936X.2020.1803961
Mali SN, Pandey A (2021) Molecular modeling studies on 2, 4-disubstituted imidazopyridines as anti-malarials: atom-based 3D-QSAR, molecular docking, virtual screening, in-silico ADMET and theoretical analysis. Journal of Computational Biophysics and Chemistry 20(03):267–282. https://doi.org/10.1142/S2737416521500125
Mishra VR, Ghanavatkar CW, Mali SN, Qureshi SI, Chaudhari HK, Sekar N (2019) Design, synthesis, antimicrobial activity and computational studies of novel azo linked substituted benzimidazole, benzoxazole and benzothiazole derivatives. Comput Biol Chem 78:330–337
Mali SN, Pandey A (2021) Balanced QSAR and molecular modeling to identify structural requirements of imidazopyridine analogues as anti-infective agents against trypanosomiases. J Comput Biophys Chem 1–32. https://doi.org/10.1142/S2737416521410015
Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu G, Lee PW, Tang Y (2012) admetSAR: a comprehensive source and free tool for assessment of chemical ADMET properties
Yap CW (2011) PaDEL-descriptor: An open source software to calculate molecular descriptors and fingerprints. J Comput Chem 32(7):1466–1474
Gramatica P, Chirico N, Papa E, Cassani S, Kovarich S (2013) QSARINS: A new software for the development, analysis, and validation of QSAR MLR models
Chirico N, Sangion A, Gramatica P, Bertato L, Casartelli I, Papa E (2021) QSARINS‐Chem standalone version: A new platform‐independent software to profile chemicals for physico‐chemical properties, fate, and toxicity. J Comput Chem
Raj KK, Manohar S, Talluri VR, Rawat DS (2015) Insights into activity enhancement of 4-aminoquinoline-based hybrids using atom-based and field-based QSAR studies. Med Chem Res 24(3):1136–1154
Zhang G, Chen Y, Liu F, Huang J, Li P, Wang B, Zhao W, Chen M, Xu S, Guan F, Tian M (2021) Comprehensive investigation of structural properties (X-ray diffraction, IR, Hirshfeld, MEP and FMOs) and in silico screening of potential biological activity of Euphorbia factor L1. J Mol Struct 1246:131237
Acknowledgements
We, the author(s) clearly indicate that some of explanations for theoretical section of this manuscript (data) has been presented previously in the conference proceeding (Section 3 (subsection 3.5 (Paragraph nos. 2–4) which can be accessed at https://sciforum.net/paper/view/11655 (13 November 2021 by MDPI in The 25th International Electronic Conference on Synthetic Organic Chemistry session Computational Chemistry).
We wish to thank the Dept. of Pharmaceutical Sciences, Birla Institute of Technology, Mesra, India, for financial assistance and Schrodinger team, Banglore, India, for providing the technical and software support to carry out the work (a trial license provided to SM). Author is also thankful for the provision of IRF (PHD/PH/10006/20) (Ref. No. GO/Estb/Ph.D./IRF/2020–21/) provided by BIT, Mesra, India, to S.M. One of the authors SM is thankful to Dr. Paola Gramatica for providing the software QSARINS-Chem 2.2.1
Funding
Financial assistance provided by Dept. of Pharmaceutical Sciences, Birla Institute of Technology, Mesra, India.
Author information
Authors and Affiliations
Contributions
All the authors read and approved the final manuscript. SM and AP: writing, designing, visualization; BT: designing and software; CL: software and interpretations.
Corresponding author
Ethics declarations
Ethical approval
None to report.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mali, S.N., Pandey, A., Thorat, B.R. et al. Multiple 3D- and 2D-quantitative structure–activity relationship models (QSAR), theoretical study and molecular modeling to identify structural requirements of imidazopyridine analogues as anti-infective agents against tuberculosis. Struct Chem 33, 679–694 (2022). https://doi.org/10.1007/s11224-022-01879-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-01879-2