Abstract
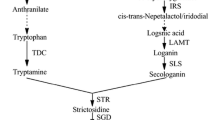
Erythroxylum coca (Erythroxylaceae) is the source of the tropane alkaloid cocaine. Several lines of evidence suggest that tropane alkaloid biosynthesis in E. coca differs from that in solanaceous species, but there are many gaps in our understanding of the pathways in both groups. The development of an E. coca cell culture that produces cocaine could provide a reproducible model system for discovering novel biosynthetic genes and study pathway regulation. Calli cultures were successfully established from young leaf explants on three different media: Anderson’s Rhododendron, Gamborg B5, and modified Murashige-Tucker, all supplemented with growth regulators: 2,4-D (0.6 mg L−1), indole butyric acid (0.06 mg L−1), and benzylaminopurine (0.5 mg L−1). All accumulated cocaine and cinnamoylcocaine at levels of 0.05–0.5 nmol per gram dry weight, as determined by LC–MS, several orders of magnitude below the concentration found in the intact plant. Anderson’s Rhododendron medium supported the highest level of tropane alkaloid production, as well as the highest level of the amino acids arginine, glutamate, proline and phenylalanine, all thought to be precursors of cocaine, but contained generally lower levels of hydroxycinnamate-quinate esters, such as chlorogenic acid. These differences may be ascribed to its relatively low content of nitrate or salts, or its high content of adenine. Addition of 100 μM salicylic acid or coronalon, an analog of the bioactive jasmonic acid-isoleucine conjugate, did not result in any increase in tropane alkaloid production. These E. coca calli could provide valuable material for studies on tropane alkaloid biosynthesis and regulation.








Similar content being viewed by others
Abbreviations
- LC–MS:
-
Liquid chromatography-mass spectrometry
- 2,4 D:
-
2,4-d-dichlorophenoxyacetic acid
- IBA:
-
Indole 3-butyric acid
- BAP:
-
Benzyl amino purine
- RH:
-
Relative humidity
References
Anderson WC (1978) Tissue culture propogation of Rhododendrons. In Vitro J Tissue Cult Assoc 14:334
Anderson JP et al (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell Online 16:3460–3479. doi:10.1105/tpc.104.025833
Baque MA, Lee EJ, Paek KY (2010) Medium salt strength induced changes in growth, physiology and secondary metabolite content in adventitious roots of Morinda citrifolia: the role of antioxidant enzymes and phenylalanine ammonia lyase. Plant Cell Rep 29:685–694. doi:10.1007/s00299-010-0854-4
Behrend J, Mateles RI (1975) Nitrogen metabolism in plant cell suspension cultures I. Effect of amino acids on growth. Plant Physiol 56:584–589. doi:10.1104/pp.56.5.584
Behrend J, Mateles RI (1976) Nitrogen metabolism in plant cell suspension cultures II. Role of organic acids during growth on ammonia. Plant Physiol 58:510–512. doi:10.1104/pp.58.4.510
Bensaddek L, Gillet F, Saucedo JEN, Fliniaux MA (2001) The effect of nitrate and ammonium concentrations on growth and alkaloid accumulation of Atropa belladonna hairy roots. J Biotechnol 85:35–40. doi:10.1016/s0168-1656(00)00372-2
Biondi S, Scaramagli S, Oksman-Caldentey KM, Poli F (2002) Secondary metabolism in root and callus cultures of Hyoscyamus muticus L.: the relationship between morphological organisation and response to methyl jasmonate. Plant Sci 163:563–569
Castaings L, Marchive C, Meyer C, Krapp A (2011) Nitrogen signalling in Arabidopsis: how to obtain insights into a complex signalling network. J Exp Bot 62:1391–1397. doi:10.1093/jxb/erq375
Catapan E, Luis M, da Silva B, Moreno FN, Viana AM (2002) Micropropagation, callus and root culture of Phyllanthus urinaria (Euphorbiaceae). Plant Cell Tissue Organ Cult 70:301–309. doi:10.1023/a:1016529110605
Champagne A, Rischer H, Oksman-Caldentey K-M, Boutry M (2012) In-depth proteome mining of cultured Catharanthus roseus cells identifies candidate proteins involved in the synthesis and transport of secondary metabolites. Proteomics 12:3536–3547. doi:10.1002/pmic.201200218
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell TissueOrgan Cult 106:279–288. doi:10.1007/s11240-011-9919-5
Demeyer K, Dejaegere R (1989) Influence of the ion-balance in the growth-medium on the yield and alkaloid content of Datura stramonium L. Plant Soil 114:289–294. doi:10.1007/bf02220809
Demeyer K, Dejaegere R (1992) Effect of the nitrogen form used in the growth medium (NO3 −, NH4 +) on alkaloid production in Datura stramonium L. Plant Soil 147:79–86. doi:10.1007/bf00009373
Desgagne-Penix I, Khan MF, Schriemer DC, Cram D, Nowak J, Facchini PJ (2010) Integration of deep transcriptome and proteome analyses reveals the components of alkaloid metabolism in opium poppy cell cultures. BMC Plant Biol 10:252. doi:10.1186/1471-2229-10-252
Dillehay TD, Rossen J, Ugent D, Karathanasis A, Vásquez V, Netherly PJ (2010) Early Holocene coca chewing in northern Peru. Antiquity 84:939–953
Docimo T, Reichelt M, Schneider B, Kai M, Kunert G, Gershenzon J, D’Auria JC (2012) The first step in the biosynthesis of cocaine in Erythroxylum coca: the characterization of arginine and ornithine decarboxylases. Plant Mol Biol 78:599–615. doi:10.1007/s11103-012-9886-1
Drager B (2002) Analysis of tropane and related alkaloids. J Chromatogr 978:1–35
Ehleringer JR, Casale JF, Lott MJ, Ford VL (2000) Tracing the geographical origin of cocaine. Nature 408:311–312
el Jaber-Vazdekis N, Barres ML, Ravelo AG, Zarate R (2008) Effects of elicitors on tropane alkaloids and gene expression in Atropa baetica transgenic hairy roots. J Nat Prod 71:2026–2031. doi:10.1021/np800573j
Facchini PJ (2001) Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Physiol Plant Mol Biol 52:29–66. doi:10.1146/annurev.arplant.52.1.29
Facchini PJ, De Luca V (2008) Opium poppy and Madagascar periwinkle: model non-model systems to investigate alkaloid biosynthesis in plants. Plant J 54:763–784. doi:10.1111/j.1365-313X.2008.03438.x
Facchini PJ, Park SU (2003) Developmental and inducible accumulation of gene transcripts involved in alkaloid biosynthesis in opium poppy. Phytochemistry 64:177–186
Facchini PJ, Johnson AG, Poupart J, DeLuca V (1996) Uncoupled defense gene expression and antimicrobial alkaloid accumulation in elicited opium poppy cell cultures. Plant Physiol 111:687–697. doi:10.1104/pp.111.3.687
Farrow SC, Hagel JM, Facchini PJ (2012) Transcript and metabolite profiling in cell cultures of 18 plant species that produce benzylisoquinoline alkaloids. Phytochemistry 77:79–88. doi:10.1016/j.phytochem.2012.02.014
Flores-Sanchez IJ, Pec J, Fei J, Choi YH, Dusek J, Verpoorte R (2009) Elicitation studies in cell suspension cultures of Cannabis sativa L. J Biotechnol 143:157–168. doi:10.1016/j.jbiotec.2009.05.006
Fodor G, Dharanipragada R (1994) Tropane Alkaloids. Nat Prod Rep 11:443–450
Ford YY, Ratcliffe RG, Robins RJ (1998) In vivo nuclear-magnetic-resonance analysis of polyamine and alkaloid metabolism in transformed root cultures of Datura stramonium L.: evidence for the involvement of putrescine in phytohormone-induced de-differentiation. Planta 205:205–213
Fritz C, Palacios-Rojas N, Feil R, Stitt M (2006) Regulation of secondary metabolism by the carbon–nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J 46:533–548. doi:10.1111/j.1365-313X.2006.02715.x
Gadzovska S, Maury S, Delaunay A, Spasenoski M, Hagege D, Courtois D, Joseph C (2013) The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult 113:25–39. doi:10.1007/s11240-012-0248-0
Gamborg OL, Shyluk JP (1970) The culture of plant cells with ammonium salts as the sole nitrogen source. Plant Physiol 45:598–600. doi:10.1104/pp.45.5.598
Gamborg OL, Murashige T, Thorpe TA, Vasil IK (1976) Plant-tissue culture media. In Vitro J Tissue Cult Assoc 12:473–478
Greenway MB, Phillips IC, Lloyd MN, Hubstenberger JF, Phillips GC (2012) A nutrient medium for diverse applications and tissue growth of plant species in vitro. In Vitro Cell Dev Biol Plant 48:403–410. doi:10.1007/s11627-012-9452-1
Griffin WJ, Lin GD (2000) Chemotaxonomy and geographical distribution of tropane alkaloids. Phytochemistry 53:623–637
Grinspoon L, Bakalar JB (1981) Coca and cocaine as medicines: an historical review. J Ethnopharmacol 3:149–159
Grynkiewicz G, Gadzikowska M (2008) Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol Rep 60:439–463
Guo Z-G, Liu Y, Gong M-Z, Chen W, Li W-Y (2013) Regulation of vinblastine biosynthesis in cell suspension cultures of Catharanthus roseus. Plant Cell Tissue Organ Cult 112:43–54. doi:10.1007/s11240-012-0213-y
Gupta KJ et al (2013) The form of nitrogen nutrition affects resistance against Pseudomonas syringae pv. phaseolicola in tobacco. J Exp Bot 64:553–568. doi:10.1093/jxb/ers348
Haider G, Kislinger T, Kutchan TM (1997) Barbiturate induced benzophenanthridine alkaloid formation proceeds by gene transcript accumulation in the California poppy. Biochem Biophys Res Commun 241:606–610. doi:10.1006/bbrc.1997.7842
Hairston NGS (1989) Ecological experiments: purpose, design, and execution. Cambridge University Press, New York
Hartmann T, Witte L, Oprach F, Toppel G (1986) Reinvestigation of the alkaloid composition of Atropa belladonna plants, root cultures, and cell-suspension cultures. Planta Med 52(5):390–395
Hashimoto T, Yamada Y (1994) Alkaloid biogenesis: molecular aspects. Annu Rev Plant Physiol Plant Mol Biol 45:257–285. doi:10.1146/annurev.arplant.45.1.257
Hauschild K, Pauli HH, Kutchan TM (1998) Isolation and analysis of a gene bbe1 encoding the berberine bridge enzyme from the California poppy Eschscholzia californica. Plant Mol Biol 36:473–478. doi:10.1023/a:1005917808232
Hsia CN, Korban SS (1997) The influence of cytokinins and ionic strength of Anderson’s medium on shoot establishment and proliferation of evergreen azalea. Euphytica 93:11–17. doi:10.1023/a:1002945922280
Huang T, Gao WY, Wang J, Cao Y, Zhao YX, Huang LQ, Liu CX (2010) Selection and optimization of a high-producing tissue culture of Panax ginseng CA Meyer. Acta Physiol Plant 32:765–772. doi:10.1007/s11738-010-0461-6
Humphrey AJ, O’Hagan D (2001) Tropane alkaloid biosynthesis. A century old problem unresolved. Nat Prod Rep 18:494–502
Jacob A, Malpathak N (2005) Manipulation of MS and B5 components for enhancement of growth and solasodine production in hairy root cultures of Solanum khasianum Clarke. Plant Cell Tissue Organ Cult 80:247–257. doi:10.1007/s11240-004-0740-2
Jirschitzka J, Schmidt GW, Reichelt M, Schneider B, Gershenzon J, D’Auria JC (2012) Plant tropane alkaloid biosynthesis evolved independently in the Solanaceae and Erythroxylaceae. Proc Natl Acad Sci 109:10304–10309. doi:10.1073/pnas.1200473109
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3(13):1222–1239
Khanam N, Khoo C, Close R, Khan AG (2000) Organogenesis, differentiation and histolocalization of alkaloids in cultured tissues and organs of Duboisia myoporoides R. Br. Ann Bot 86:745–752. doi:10.1006/anbo.2000.1247
Kim YD, Kang SM, Min JY, Choi WK, Jeong MJ, Karigar CS, Choi MS (2010) Production of tropane alkaloids during de-differentiation of Scopolia parviflora calli. J Nat Prod 73:147–150. doi:10.1021/np900525n
Kováčik J, Klejdus B, Babula P, Jarošová M (2014) Variation of antioxidants and secondary metabolites in nitrogen-deficient barley plants. J Plant Physiol 171:260–268. doi:10.1016/j.jplph.2013.08.004
Krouk G, Gojon A (2011) An original ion (nitrate) sensing mechanism: story of the discovery in plants and perspectives. Med Sci 27:1045–1047. doi:10.1051/medsci/20112712002
Krouk G, Mirowski P, LeCun Y, Shasha DE, Coruzzi GM (2010) Predictive network modeling of the high-resolution dynamic plant transcriptome in response to nitrate. Gen Biol 11:R123. doi:10.1186/gb-2010-11-12-r123
Leete E (1980) Biosynthesis of cocaine and cuscohygrine in Erythroxylon coca. J Chem Soc Chem Commun 23:1170–1171. doi:10.1039/c39800001170
Liscombe DK, Macleod BP, Loukanina N, Nandi OI, Facchini PJ (2005) Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms. Phytochemistry 66:1374–1393
Lounasmaa M, Tamminen T (1993) The tropane alkaloids. In: Cordell GA (ed) The alkaloids, vol 44. Academic Press, New York, pp 1–114
Lydon J, Zimmerman RH, Fordham IM, Lusby WR (1993) Tissue culture and alkaloid production of Erthroxylum coca var. coca. J Herbs Spices Med Plants 2:3–14
Medinabolivar F, Flores HE (1995) Selection for hyoscyamine and cinnamoyl putrescine overproduction in cell and root cultures of Hyoscyamus muticus. Plant Physiol 108:1553–1560
Merrett MJ, Handoll C (1967) Some effects of adenine on growth, respiration, adenosine diphosphate and triphosphate concentrations in plant tissues. New Phytol 66:569–575. doi:10.1111/j.1469-8137.1967.tb05429.x
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Murashige T, Tucker DPH (1962) Growth factor requirements of citrus tissue culture. In: Proceedings of the 1st international citrus symposium, pp 1155–1161
Oksman-Caldentey KM, Arroo R (2000) Regulation of tropane alkaloid metabolism in plants and plant cell cultures. In: Verpoorte R, Alfermann A (eds) Metabolic engineering of plant secondary metabolism. Kluwer Academic Publishers, Dordrecht, pp 253–281
Palacio L, Cantero JJ, Cusido RM, Goleniowski ME (2012) Phenolic compound production in relation to differentiation in cell and tissue cultures of Larrea divaricata (Cav.). Plant Sci 193:1–7. doi:10.1016/j.plantsci.2012.05.007
Palazon J, Altabella T, Cusido R, Ribo M, Pinol MT (1995) Growth and tropane alkaloid production in agrobacterium transformed roots and derived callus of Datura. Biol Plant 37:161–168. doi:10.1007/bf02913204
Palazon J, Navarro-Ocana A, Hernandez-Vazquez L, Mirjalili MH (2008) Application of metabolic engineering to the production of scopolamine. Molecules 13:1722–1742. doi:10.3390/molecules13081722
Pardo Torre JC, Schmidt GW, Paetz C, Reichelt M, Schneider B, Gershenzon J, D’Auria JC (2013) The biosynthesis of hydroxycinnamoyl quinate esters and their role in the storage of cocaine in Erythroxylum coca. Phytochemistry 91:177–186. doi:10.1016/j.phytochem.2012.09.009
Pec J, Flores-Sanchez IJ, Choi YH, Verpoorte R (2010) Metabolic analysis of elicited cell suspension cultures of Cannabis sativa L. by H-1-NMR spectroscopy. Biotechnol Lett 32:935–941. doi:10.1007/s10529-010-0225-9
Plowman T, Hensold N (2004) Names, types and distribution of neotropical species of Erythroxylum (Erythroxylaceae). Brittonia 56:1–53
Plowman T, Rivier L (1983) Cocaine and cinnamoylcocaine content of Erythroxylum species. Ann Bot 51:641–659
Premkumar G, Sankaranarayanan R, Jeeva S, Rajarathinam K (2011) Cytokinin induced shoot regeneration and flowering of Scoparia dulcis L. (Scrophulariaceae)–an ethnomedicinal herb Asian Pacific. J Trop Biomed 1:169–172. doi:10.1016/S2221-1691(11)60020-8
Rischer H et al (2006) Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc Natl Acad Sci USA 103:5614–5619. doi:10.1073/pnas.0601027103
Ruetsch YA, Boni T, Borgeat A (2001) From cocaine to ropivacaine: the history of local anesthetic drugs. Curr Top Med Chem 1:175–182
Scheible W-R et al (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136:2483–2499. doi:10.1104/pp.104.047019
Schuler G et al (2004) Coronalon: a powerful tool in plant stress physiology. FEBS Lett 563:17–22. doi:10.1016/s0014-5793(04)00239-x
Sokal RR, Rohlf J (1982) Biometry: the principles and practice of statistics in biological research. In: Freeman WH (ed) Current contents/agriculture biology & environmental sciences, vol 41, W.H. Freeman, New York
Takahashi H, Kanayama Y, Zheng MS, Kusano T, Hase S, Ikegami M, Shah J (2004) Antagonistic interactions between the SA and JA signaling pathways in Arabidopsis modulate expression of defense genes and gene-for-gene resistance to cucumber mosaic virus. Plant Cell Physiol 45:803–809. doi:10.1093/pcp/pch085
ten Hoopen HJG, Vinke JL, Moreno PRH, Verpoorte R, Heijnen JJ (2002) Influence of temperature on growth and ajmalicine production by Catharantus roseus suspension cultures. Enzyme Microb Technol 30:56–65. doi:10.1016/s0141-0229(01)00456-2
Wang H, Lu YQ, Liu P, Wen W, Zhang JH, Ge XC, Xia Y (2013) The ammonium/nitrate ratio is an input signal in the temperature-modulated, SNC1-mediated and EDS1-dependent autoimmunity of nudt6-2 nudt7. Plant J 73:262–275. doi:10.1111/tpj.12032
Weil AT (1980) Cocoa, not cocaine. Science 209:1182
Wu C-H, Dewir YH, Hahn E-J, Paek K-Y (2006) Optimization of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia J. Plant Biol 49:193–199
Yamada Y, Endo T (1984) Tropane alkaloid production in cultured-cells of Duboisia leichhardtii. Plant Cell Rep 3:186–188
Yukimune Y, Tabata H, Higashi Y, Hara Y (1996) Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat Biotechnol 14:1129–1132. doi:10.1038/nbt0996-1129
Yukimune Y, Hara Y, Nomura E, Seto H, Yoshida S (2000) The configuration of methyl jasmonate affects paclitaxel and baccatin III production in Taxus cells. Phytochemistry 54:13–17. doi:10.1016/s0031-9422(00)00006-6
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333. doi:10.1016/j.biotechadv.2005.01.003
Zhou ML, Shao JR, Tang YX (2009) Production and metabolic engineering of terpenoid indole alkaloids in cell cultures of the medicinal plant Catharanthus roseus (L.) G. Don (Madagascar periwinkle). Biotechnol Appl Biochem 52:313–323. doi:10.1042/ba20080239
Ziegler J, Facchini PJ (2008) Alkaloid biosynthesis: metabolism and trafficking. Annu Rev Plant Biol 59:735–769. doi:10.1146/annurev.arplant.59.032607.092730
Acknowledgments
This work was supported by the Max Planck Society and an Alexander von Humboldt Foundation postdoctoral fellowship to J.D. We thank Dr. Tamara Krügel for her advice regarding tissue culture methods and practices, Andreas Weber and the rest of the gardening staff of the MPI-ICE for their help in plant maintenance, Dr. Axel Mithöfer for providing the coronalon used in our elicitation experiments, and Jan Jirschitzka for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Docimo, T., Davis, A.J., Luck, K. et al. Influence of medium and elicitors on the production of cocaine, amino acids and phytohormones by Erythroxylum coca calli. Plant Cell Tiss Organ Cult 120, 1061–1075 (2015). https://doi.org/10.1007/s11240-014-0660-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0660-8