Abstract
This study is a continuation of our previous work, which focused on a near-wellbore water blockage alleviation by applying a thermally cured silane-functionalized benzoxazine to modify rock wettability. In this new analysis, we have demonstrated that the resin can be applied in low-permeability sandstones (approximately 15 mD as opposed to 100 to 200 mD in the previous study) to change the rock surface wettability from water-wet to intermediate gas-wet. We have also demonstrated that curing temperatures as low as 125 °C (as opposed to 180 °C in our previous study) can significantly change wettability, indicating surface functionalization through the silane moiety and ring-opening polymerization of the benzoxazine moiety. In drainage core flooding experiments at 2.5 wt.% resin loading, compared to untreated samples, brine recovery increments of 6.3 to 6.9% were obtained for curing temperatures of 125 to 180 °C, respectively. A maximum 20% increment in the end-point relative gas permeability was achieved at a curing temperature of 180 °C. A coupled experimental and numerical study, conducted at core and wellbore scales, demonstrates the potential effectiveness of our chemical treatment in improving gas productivity at the field scale. Reservoir simulations indicate a 2.9 to 10.6% improvement in gas deliverability for a treatment radius of 4 to 16 m, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The transient reduction in global energy and hydrocarbon demand drastically affected energy prices in the first half of 2020. Combined with renewed efforts toward addressing both climate change and economic recovery of the industrial sector, natural gas is poised to replace coal in the future as a less carbon-intensive fuel and as a transition to renewable energy sources. (IEA 2020) However, this approach is predicated on the industry’s access to viable and economical gas reservoirs. With the growing trend of finding much-needed gas reserves in low-permeability rock formations, the industry is facing significant challenges with securing adequate gas deliverability over the intermediate to long term. For example, one of the most challenging issues associated with low-permeability reservoirs is a continuous increment of the capillary strength present in their significantly finer pores. (Bennion et al. 2000a) In such formations, when water invades the wellbore region, capillary effects lead to strong water imbibition resulting in blockage and reduced gas deliverability. This invading water mainly accumulates in the small pores of the predominantly water-wet reservoir, causing a moderate to substantial reduction in the relative permeability to gas. (Bahrami et al. 2011; Bennion et al. 1996b; Deng and King 2018; Eakin et al. 1965; Holditch 1979; Mirzaei Paiaman et al. 2010; Parekh and Sharma 2004).
The damage associated with water blockage in low-permeability reservoirs occurs most often following mechanical interventions, production stimulation, and certain other reservoir events (Bennion et al. 1996b). The fluid phase used during drilling and workover operations, even when underbalanced conditions are applied, can create appreciable damage around the wellbore due to the strong capillary strength of low-permeability formations (Khansari 2009). During hydraulic fracturing operations, considerable quantities of fluids invade the rock pores, negatively offsetting the gas deliverability enhancement sought from the fracturing operation (Bahrami et al. 2011; Bennion et al. 1996a; Parekh and Sharma 2004). Gas relative permeability can also decrease as a consequence of water crossflow from other reservoir layers reaching the gas-producing intervals (Khan et al. 2017). Water coning, in combination with the effects of reservoir heterogeneity, can also affect well deliverability due to formation damage. The above effects are not limited to mature fields as water influx could arrive at production intervals in an early stage of the reservoir life (Armenta and Wojtanowicz 2002). In some instances, alcohol injection around the wellbore can decrease the interfacial tension of the water phase, which can help to alleviate the blockage; nevertheless, this would be a temporary measure and often requires recurrent wellbore interventions, which, even if economic factors are ignored, could further damage the wellbore if the blockage persists.
During the last few decades, several researchers have examined alternative remedial techniques by developing permanent treatments that can decrease the water trapping effect in low-permeability sandstone through wettability alteration. This effect is primarily dominated by implementing chemical treatment that targets the surface free energy of the rock pore surface (i.e., sandstone); ideally, after treatment with a chemical solution, the wettability is permanently modified, causing a reduction in the capillary strength (Drummond and Israelachvili 2002; Gupta and Mohanty 2011; Park and Seo 2011). In this case, for a sandstone, an increase in water contact angle following rock treatment indicates a wettability variation from strongly water-wet to intermediate gas-wet (with the nature and extent of the variations depending on the treatment’s specific circumstances). Moreover, the liquid blockage around the wellbore area becomes more dramatic in gas-condensate reservoirs when bottom hole pressure falls below dew point pressure creating a condensate build up that permanently blocks the pore system in the area around the wellbore (Al-Anazi et al. 2005) (Bang et al. 2009; Noh and Firoozabadi 2008). The presence of the condensate phase in gas reservoirs can lower the relative permeability to gas by more than 90% leading to a drastic gas deliverability reduction (Bang et al. 2009).
In early attempts at the above approach, Fahes and Firoozabadi pioneered the use of fluorinated compounds to alter rock wettability from liquid-wet to intermediate-wet. Initially, they used anionic fluoropolymers (FC-759 and FC-722) to achieve hydrophobic and oleophobic conditions in low-permeability sandstone samples by evaluating the increase in the decane-rock contact angle from 0° to 60° (Fahes and Firoozabadi 2005; Li and Firoozabadi 2000). Some of the fluorinated compounds used contain silane moieties that can covalently bond to the sandstone pore surface, thus immobilizing the polymer and making it a more permanent treatment. These researched obtained favorable wettability alteration even under high-temperature conditions of 140 °C. They evaluated that the effect of chemical treatment concentration on rock permeability was evaluated showing 4 and 8% concentrations resulted in a 10 and 80% permeability reduction, respectively. The large reduction at the higher concentration is due generally to a decrease in the effective pore diameter and not just a wettability alteration. Other researchers have shown that treating rock samples with alcohol-based fluorocarbon chemicals can result in wettability changes to hydrophobic (to prevent water blocking) or oleophobic (to prevent condensate block) condition generated by the wettability alteration (Li et al. 2011; Noh and Firoozabadi 2008; Zhang et al. 2014) and that alcohol functionalities seemed to improve the durability of the rock treatment. After the introduction of commercial perfluoroosilanes in the early 2000s, Sharifzade et al. experimented with a sol–gel reaction between perfluorodecylsilane (FAS17) and tetraethoxysilane (TEOS) to covalently bond the perfluorochain to the rock surface and change the surface wettability of the pores. Others have reached super-hydrophobic and oleophobic conditions over sandstone samples by the use of fluorinated nano-components (Franco-Aguirre et al. 2018; Jin et al. 2016). For example, Mousavi et al. obtained super-hydrophobic conditions by the use of nanostructures synthesized by the reaction of nonafluorohydroxyltriethoxy silane (FAS9) and tetraethoxysilane (TEOS) resulting in a decane contact angle on a carbonate surface of approximately 61°. The use of a perfluorooctyltriclhlorosilane (FAS12) and TEOS reaction reached superoleophobic conditions resulting from surface roughness alteration as well.
As revealed by the above studies, fluorinated components can create ideal hydrophobic, oleophobic or even super-hydrophobic and superolephobic conditions over the rock surface; nonetheless, most of them represent a significant environmental concern. For instance, long-chain fluoroalkanes are very stable due to the strength of the molecular carbon–fluorine bonds; however, they exhibit environmental toxicity, tend to bio-accumulate, and do not readily biodegrade. (Park et al. 2013; Rice 2015; Winkens et al. 2017) Additionally, the complexity of the process associated with the chemical synthesis of a fluorocarbon chemical increases its cost making; it is likely to be cost-prohibitive at the scales necessary for field deployment in a gas reservoir.
In our previous study, we demonstrated the performance of benzoxazine monomer to alter wettability from strongly water-wet to the intermediate gas-wet condition (Mauricio Ceron Lopez et al. 2021). Sandstone samples (i.e., Berea Upper Gray (BUG)) with a moderate absolute permeability value of about 130 mD were treated with the silane-containing benzoxazine resin monomer, which was then cured (or polymerized) at 180 °C. This material is particularly advantageous as the silane moiety can react with the surface of a sandstone thus covalently immobilizing it. As proposed in the literature, high-temperature curing is ideal for reducing the surface free energy of substrates treated with this class of monomer (Gogoi et al. 2014; Liao et al. 2008; Liu et al. 2013; Wang et al. 2006, 2011a). In our previous study, these comparatively high permeability samples showed an increase of 22% in gas relative permeability, and a decrease of up 10% in water saturation was observed after drainage core flooding experiments. However, in some circumstances (if not most), it might not be practical or feasible economically to have or obtain such high-temperature conditions around the wellbore.
Several researchers have applied different types of benzoxazine resin to alter the wettability of different materials such as glass, cotton, and silicon wafers (Gogoi et al. 2014; Liao et al. 2008; Liu et al. 2013; Wang et al. 2006, 2011a). They obtained hydrophobic conditions after treating those materials at a range of curing temperatures; nevertheless, they determined that the water contact angle decreases with the temperature reduction (i.e., the extent of the polymerization diminished with temperature decrease). Often, the curing temperature exceeds the temperature limit of the material where the substrate may not be stable at these relatively high temperatures. Moreover, there is not enough evidence in the literature explaining the curing temperature effect on the achievable wettability alteration in porous materials such as rock samples. As a continuation of our previous work, in this study, we determined the efficacy of this method in low-permeability (approximately 10 to 20 mD) sandstone samples more representative of low-permeability reservoirs where water blockage is more common. Furthermore, the effect of chemical concentration (2.5 and 8 wt.%) and polymerization temperature (110, 125, 150, and 180 °C) was evaluated when treating sandstone samples. The wider temperature range used in this study is representative of a more extensive set of gas reservoirs.
Recognizing that a high monomer concentration could negatively impact permeability through a reduction in porosity, a brief chemical concentration screening technique was employed to find a compromise between wettability alteration and impact on absolute gas permeability. With an appropriate concentration chosen, various temperatures and curing times were used (recognizing that lower temperatures required longer curing times which is not an issue given that these resins would be subjected to prolonged curing conditions in the subsurface) to optimize the contact angle/wettability alteration. These tests generally depicted intermediate gas-wet conditions. A spontaneous imbibition testing procedure was then performed to assess the effect of the chemical over capillary imbibition pressure, determining the amount of water that would invade the porous space of the rock. Finally, core-flooding experiments were performed at elevated pressure and temperature to determine the induced variation in the differential pressure and water recovery profiles.
Subsequently, the outputs of the above core flooding experiments (along with those from our previous study (Mauricio Ceron Lopez et al. 2021)) using Sendra software (Prores AS) were history matched using a 1D core-scale simulation technique to obtain the relative permeability curve before and after treatment. This procedure provides a realistic understanding of the effect of rock permeability when water blockage occurs and how a benzoxazine monomer can improve deliverability by shifting wettability. We then use these data in a numerical box model of the near-wellbore region in a reservoir to understand the impact of our proposed treatment on water saturation and gas deliverability. The effects of different absolute rock permeabilities and different treatment radii around the wellbore were assessed to evaluate the efficacy of benzoxazine resin treatment in mitigating water blockage in gas reservoirs under a range of application conditions.
2 Materials, Experimental Details and Numerical Simulation Methods
2.1 Materials
The proposed benzoxazine monomer was synthesized using p-cresol, paraformaldehyde, and 3-amipropyltrimethoxysilane, following the steps available in the literature (Liu et al. 2013; Lopez et al. 2021). This monomer’s silane moiety is covalently attached to the rock surface via a silylation reaction (Arjomand et al. 2020). Furthermore, readily available and inexpensive alcohol solvent (i.e., isopropanol) was used to dilute the monomer for injection into the rock samples for treatment. In our previous work (Lopez et al. 2021), the application of 8–10 wt. % benzoxazine solution in a sandstone sample (approximately permeability of 150 md) was able to alter rock wettability to intermediate-wet conditions. For this study, we used lower permeability samples (i.e., 5 to 25 mD). Therefore, a more suitable benzoxazine concentration was analyzed to minimize possible blockage or porous space reduction.
Before and after rock treatment, the absolute permeability variation was observed in Carbon Tan sandstone samples (15–20 md). The low-permeability CT samples were sourced from a commercial distributor (Kocurek Industries, USA). They were cleaned in a temperature-controlled Dean-Stark setup with methanol and toluene to remove any possible contaminants present in their pore space and then dried in an oven at 70 °C for 24 h. Porosity and permeability data were measured on cleaned and dried samples using an automated gas porosi-permeameter(Arjomand et al. 2020). It is worth noting that contact angle measurements used thin rock disks (~ 5 mm thick) cut from the end of some core samples, which were cleaned and dried using a similar technique as described above. Table 1 also details the treatment applied to each of the tested samples. A relevant description of these data will be provided in the upcoming sections of the manuscript.
For all experiments involving brine, a synthetic brine was prepared using 15.5 g NaCl in 1 L of deionized water (1.5 wt.% concentration of salt). In line with our previous work, an air-brine system was used to perform the spontaneous imbibition tests and contact angle measurements. Similarly, nitrogen was used to simulate the gas phase during core flooding tests to determine the effect of the chemical treatment on the multiphase flow characteristics of the system. The core flooding experiments were conducted under the same elevated pressure and temperature conditions used in our previous work (i.e., 10.35 MPa and 60 °C).
2.2 Chemical Functionalisation
When it comes to the chemical concentration used to treat core samples, there is a balance between the level of surface wettability change (i.e., higher concentrations will have better coverage and a more wettability change) and the effect on absolute permeability (i.e., higher concentrations will start to fill the rock pore space leading to a degradation in absolute permeability). As a result, it is required to find a compromise on the concentration that would have balanced effects. Therefore, to determine the monomer concentration more suited for the treatment of low-permeability samples, two different chemical solutions with 8 and 2.5 wt.% concentrations were used to treat samples CT1 and CT2-CT5, respectively (Table 1). The first concentration is based on the results obtained during our previous analysis, while a lower concentration of 2.5% was determined that can mitigate the effects of pore diameter alteration. The core saturation and treatment process are detailed in our previous study (Mauricio Ceron Lopez et al. 2021). Subsequently, for samples CT2-CT4, curing temperatures ranging from 125 to 180 °C were used over curing times of 2 h. For sample CT5, we explored whether an extending curing time could balance out the lower curing temperature of 110 °C and yield desirable results. Following the curing process, rock samples were subjected to Dean-Stark extraction with methanol for 24 h to remove any polymeric material that did not react with the rock surface and remained loose in the pores. The cleaned samples were dried in the oven at 70 °C for 24 h before performing the planned laboratory evaluations. The impact of the chemical concentration over absolute permeability was determined, and then after choosing the more suited concentration (i.e., having a lower negative impact on permeability), the effect of different curing temperatures over the possible wettability alteration was evaluated using contact angle measurements conducted on rock substrates, as well as spontaneous imbibition and core flooding tests performed on core samples. The procedures for contact angle measurements, spontaneous imbibition tests, and core flooding experiments were identical to those used in our previous study. The use of identical procedures allowed for the comparison of results (Lopez et al. 2021); the procedures for the spontaneous imbibition and core flooding experiments are briefly summarized below.
2.3 Spontaneous Brine Imbibition Experiments
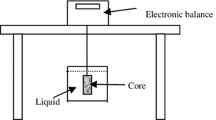
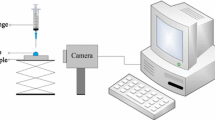
Contact angle measurements and spontaneous imbibition experiments were conducted at ambient conditions (i.e., 0.1 MPa and 25 °C) for initial assessment of the wettability alteration over the sandstone rock samples. The rock–fluid interaction was set up as a water–air-rock system to physically observe and determine the contact angle and the volume of brine (1.5 wt.% NaCl concentration) spontaneously imbibed by the rock sample. A sessile drop arrangement was used to obtain optical measurements from a high-resolution camera (see Fig. 1a) to analyze the contact points between the fluid system interaction and the untreated rock surface. Later, the rock samples were treated with the chemical solution followed by a curing process in the oven at different temperatures and times, as shown in Table 1. After the polymerisation treatment of the rock samples, contact angle measurements were repeated 10 times to obtain an average result for each sample. Once initial wettability analysis was obtained, it was required to assess the effect of the chemical solution in the porous space. Therefore, spontaneous imbibition analysis, using a high-resolution balance to record the weight of the rock sample when immersed in a beaker filled with water, was performed using untreated and treated rock samples, as shown in Fig. 1b.
2.4 Drainage Core Flooding Experiments
Core-flooding experiments were conducted on three different Carbon Tan samples to assess the effect of wettability alteration (and inevitable permeability changes) on multiphase flow characteristics of the fluid-rock system after chemical treatment. Figure 2 provides a schematic of the core-flooding system used in this research. The flooding procedure was performed at 60 °C, 10.4 MPa pore pressure, and 17.2 MPa confining pressure. The reservoir conditions were selected to assess dynamic conditions before and after treatment, considering that the chemical is initially tested at static conditions at elevated temperatures from 120 to 180 °C. Therefore, the chemical resistance is not a limitation as it can work adequately below 180 °C; we mainly focused on assessing the dynamic conditions of these core flooding conditions. Extensive details about the core-flooding procedure used can be found in our previous published work (Lopez et al. 2021).
2.5 Core-scale Numerical Simulations
At the core scale, a 1D numerical model was built for each core sample with 100 grid cells along its axis, having overall dimensions of 0.076 m (length) by 0.038 m (diameter). In this grid system, a drainage process was then simulated in which nitrogen gas displaced the fully brine-saturated core. Once initialized, a water production and differential pressure history matching was performed on the experimentally measured data using a rigorous iterative procedure in Sendra v2018.1 (Prores AS). The iterative procedure follows the equations and rules set in various analytical relative permeability models (e.g., proposed by Corey, Sigmund & McCaffery). To ascertain the effects of the benzoxazine treatment on the relative permeability characteristics, history matching was conducted on the differential pressure and water recovery data from the drainage core-flooding experiment (from cores before and after chemical treatment). We have also incorporated the data from our previous study (Lopez et al. 2021), which examined the effects of benzoxazine treatment on higher permeability samples (i.e., BUG samples of approximately 130 mD) to allow comparison with the results of this study which focuses on low-permeability cores. In our case and under both sample conditions, the Sigmund & McCaffery model provided a very close fit generating the relative permeability curves as the output. These data were then extended into the numerical reservoir simulator to build the box model that allowed us to assess the effect of wettability alteration treatment on gas deliverability at a wellbore scale.
2.6 Wellbore-scale Numerical Simulations
A homogeneous box model with overall dimensions of 1219 × 1219 × 26 m (in x, y, and z directions, respectively) representing the initial wellbore conditions (with the effects of water blockage) was created using a commercial numerical reservoir simulator (T-Navigator v20.1). A grid of 100 × 100 × 8 cells (in x, y, and z directions, respectively) was generated to populate with different rock and fluid properties (e.g., porosity, permeability, initial water saturation). Moreover, local grid refinement was applied around the well trajectory to better model the fluid characteristics and pressure drawdown around the wellbore (Table 2).
The in situ conditions of the Paddy sandstone member, a relatively low-permeability member (< 100mD) of the Peace River Formation in the northwestern part of Alberta – Canada, were selected for this case study. Furthermore this formation has a mineralogy (i.e., approximately 64 % quartz) and a low permeability very similar to the Carbon Tan rock samples (Diana 2013). The Paddy member is a quartz arenite sandstone rock composed of more than 40% quartz, formed by transported and deposited sediments in an estuarine bay complex (Leckie 1994). Average porosity, depth, reservoir thickness, and pressure from the Paddy formation were considered during the laboratory analysis to reproduce the sensitivity of this formation for aqueous fluid invasion (Bennion et al. 1994; Cimolai et al. 1993). The mentioned parameters were adopted to generate the initial conditions in a vertically homogeneous and radially symmetric wellbore model, as shown in Table 3.
Water injection was simulated at the early stage of production, intending to reproduce the condition created by the invasion of the water phase into the wellbore region. For sensitivity analysis, three invasion radii of 4, 10, and 16 m were considered while using Eq. 1 to calculate the amount of chemical solution to be injected. This volume is directly proportional to the square difference of the invasion diameter (dinv) and wellbore diameter (dwell) at a determined reservoir thickness (Z). For every simulation scenario, the invasion radius defines the region or area over which the relative permeability properties are altered, while the rest of the reservoir would maintain its initial value. This arrangement reflects that water blockage occurs only in the vicinity of the wellbore. Therefore, three relative permeability cases and water saturation levels were created to represent the initial condition of the reservoir and the altered zone region effect of water blockage. Figure 3 shows the water saturation model principally characterizing the area around the wellbore with a water saturation value of 80% (Swc), while the rest of the reservoir has an initial water saturation of 40% (Swi), which is a typical irreducible water saturation as reported in the literature (Bennion et al. 2000a).
After the well setup, different production simulation scenarios were established at a constant flow rate of 1500 Mscf/day for thirty months. The minimum bottom hole flowing pressure was set at 10 MPa. The entire reservoir interval (25 m) was considered open to flow. Broadly categorized, we considered two sets of scenarios in our box model simulation runs, one with and one without the water blockage effect, with details of each set presented below:
2.6.1 Initial Reservoir Scenario
This is an ideal scenario representing the initial conditions of the reservoir with no water blockage effect considered. Accurately measuring initial water saturation is a complex challenge requiring special coring techniques using hydrocarbon or water-based muds with some chemical tracers. Benion defined a permeability and initial water saturation relationship with the aqueous phase trap index (APT), which mainly defines the trapping sensitivity effect. An APT between 0.8 and 1 is a zone of potential damage; therefore, a value of 1 is assumed for initial water saturation (Swi) (Eq. 2) and relative permeability to gas (krg) (Eq. 3) (Bennion et al. 1996c).
where krg' is the relative permeability end-point of the non-wetting phase, which is assumed to be double the relative permeability end-point measured in the laboratory before the rock treatment (Bennion et al. 1996c, 2000a, b). This was done by simply extrapolating the relative permeability to gas before treatment as determined experimentally. The m value represents the relative permeability shape factor, which is typically a value higher than 1. The Sn value represents the normalized water saturation, Swmax is the maximum water saturation value, and Swmin is the minimum water saturation. Additionally, when modeling the low-permeability scenario, the calculated initial water saturation has been increased by 25% to simulate a pessimistic scenario, which will prevent us from overestimating the final result.
2.6.2 Water Invasion Scenarios
Three well production scenarios examining the water blockage effect with different benzoxazine treatment radii (of 4, 10, and 16 m) were simulated with identical initial saturation conditions. Figure 4 shows a schematic of the different scenarios with the corresponding water saturation conditions. Similar scenario setups were then used to illustrate the enhanced productivity effect of the wettability alteration caused by benzoxazine treatment. The main input data in simulating the above scenarios were the relative permeability curves obtained from the 1D core-scale analysis to upscale the experimental core-flooding results to the wellbore box model.
It is worth noting that different permeability values of 15 mD (this study) and 130 mD (our previous study (Lopez et al. 2021)) were used in separate models to investigate the effect of absolute permeability on the achievable productivity enhancement resulting from wettability alteration.
3 Results and Discussion
This section describes the most relevant characteristics of the benzoxazine treatment at different static and dynamic conditions. Initially, the chemical polymerization temperature was tested between 120 and 180 °C, and ambient condition contact angle measurements were conducted to optimize the curing temperature/monomer concentration. Spontaneous imbibition experiments were also conducted to evaluate these effects as well on rock samples with initial permeability values less than 20 mD. Following this, core flooding experiments using realistic reservoir conditions (e.g., low permeability, low chemical concentration) were conducted at elevated pressure/temperature values. Following this, a wellbore model was built to assess the effect of the chemical treatment around the wellbore area at different treatment conditions, such as invasion radius and rock permeability. The effect of chemical concentration on rock permeability was also evaluated.
In our previous study (Lopez et al. 2021), which used moderate permeability BUG sandstone (~ 130 mD), a 10 wt.% solution of benzoxazine resin monomer was applied using a curing temperature of 180 °C. Given the reasonable permeability of these samples, there was less concern around permeability loss due to treatment. As reported, our investigation revealed only a very subtle (on average ~ 5%) decrease in sample permeabilities after treatment. However, given the low permeability of CT samples used in the current study, there was a genuine concern about the significant effect of treatment on sample permeabilities if the same high concentration was used here. Therefore, it was deemed necessary to optimize the chemical concentration so we would strike a balance between adequate surface coverage to induce necessary wettability alteration and the negative effect on permeability.
A detailed description of how our novel benzoxazine would work was provided in our previous work (Lopez et al. 2021). However, in brief, at an elevated temperature (i.e., 180 °C or lower, as examined later in this manuscript), the benzoxazine monomer structure undergoes ring-opening polymerization leading to the formation of intramolecular forces between polymer chains, thus decreasing the surface energy of the rock and the associated capillary strength of the pores. This effect can be manifested in a reduction in the rock’s spontaneous brine imbibition rate, as discussed in more detail in our previous study. Therefore, a decision was made first to perform a preliminary investigation into the effectiveness of a reduced benzoxazine concentration (i.e., 2.5 wt.%) in changing spontaneous imbibition characteristics of the CT sandstone when compared with that of a much higher concentration (i.e., 8%). Spontaneous imbibition experiments were conducted over a longer period of time relative to the contact angle measurements (time duration 30 min to 1 h). In doing the above evaluation because, as discussed in our previous work (Lopez et al. 2021), the former technique is done at the larger core scale as compared with contact angles that may be considered highly localized property of a surface conducted over a very small surface portion of a substrate. Furthermore, the spontaneous imbibition process can also be considered much more insightful in relating to the dynamics of the rock–fluid system as they are impacted by the pore network and wide pore size distribution in a core sample.
Initially, samples CT1 and CT4 were treated with 8 and 2.5 wt.% monomer concentrations, respectively, while curing conditions remained similar (180 °C for 1 h). The purpose of using this considerable difference in benzoxazine concentration was first to compare the effect of a high benzoxazine concentration value (closely similar to the concentration used in our previous research analysis, as well as other researchers’ chemical concentration used for rock sample treatment) versus a low concentration, which can avoid a possible pore diameter reduction due to chemical adsorption and presence in the porous space. As evident from the data plotted in Fig. 5, both samples behaved similarly regarding the rate and eventual level of water that spontaneously imbibed into their pore spaces before treatment. However, as expected, the treated sample CT1 exhibited a considerably larger reduction in brine imbibition rate in comparison with CT4. Furthermore, when the samples reached equilibrium, CT4 had imbibed four times more water than CT1 (see Fig. 5). These results suggest that the degree of wettability alteration is directly proportional to the chemical concentration present in the solution. Nevertheless, the result obtained for sample CT4 was still considered positively significant, as will be demonstrated further by the results of the core flooding experiments discussed later. Therefore, our attention was shifted to the effect of the chemical concentration on the pore network and the absolute permeability of CT sandstone.
With the positive effects of the chemical treatment on the spontaneous imbibition results being evident at the proposed chemical concentrations, we evaluated the permeability loss associated with both concentrations in samples CT1 and CT4. As evident from data presented in Table 1, 8 wt.% concentration reduced CT1 permeability from 21.1 to 16.2 mD, translated to 22.2% of its initial permeability, which is a considerably unacceptable amount for an already low-permeability rock. The significance of the above reduction may become more apparent, knowing that in our previous work, a 10 wt.% concentration tried with moderate permeability rocks (~ 130 mD) only induced a 2.3–6.2% permeability reduction. In comparison, the 2.5 wt.% concentration caused only a 10.7% reduction in the permeability of sample CT4, which shows negligible results of permeability reduction that would not mean a drastic alteration of the pore diameter in size reduction/increment as previously analyzed by other researchers (Fahes and Firoozabadi 2005; Noh and Firoozabadi 2008). They found a maximum absolute permeability reduction of around 8 to 19% in rock samples with absolute gas permeabilities between 2 and 10 mD after chemical treatment with a fluorinated concentration range between 4 and 8 wt.%. Fahes et al. 2005 discarded samples treated with 8% fluorinated chemical solution due to a drastic permeability reduction of around 80%. The high amount of the chemical present in the pore system could drastically alter the flowing conditions through the rock sample. Furthermore, Noh et al. 2008 discarded rock samples treated with 2 to 4 wt.% fluorinated chemical due to a more than 22% absolute permeability reduction after rock treatment. Therefore, in this study, the most suitable benzoxazine concentration during rock treatment was established as 2.5 wt.% to maintain a lower than 10% permeability reduction, to minimize the effect of chemical polymerization in the pore system by any possible blockage of the pore throat or reduction of the pore diameter in Carbon Tan rock samples between 10 and 20 mD. After thermal polymerization, rock samples were washed with a solvent (e.g., methanol) followed by a substantial water displacement at high pressure of 10 MPa, to minimize the presence of the remaining polymer that did not create a covalent bond with the surface of the rock.
3.1 Effect of Curing Temperature on Contact Angle and Spontaneous Imbibition Results
After determining that a 2.5 wt.% treatment solution would produce acceptable wettability alteration without a significant adverse effect on rock permeability, our attention turned to the possibility of reducing the curing temperature, which would expand the application envelope of our novel benzoxazine treatment to a lower-temperature (e.g., shallower) reservoirs. This section of the manuscript will present and discuss the results of the contact angle measurements and spontaneous imbibition tests performed in pursuing the above possibility.
Based on our previous study, contact angle values between 90° and 110° could be achieved, indicating intermediate wettability conditions and the ability to alleviate part of the damage associated with water blockage (Mauricio Ceron Lopez et al. 2021). Furthermore, values in this range are consistent with those obtained for the same resin cured on glass surfaces (Liu et al. 2013). Table 4 shows the average water contact angle measured under ambient conditions over substrates cut from rock samples CT2, CT3, and CT4 that were all treated using 2.5 wt.% concentration but at 125, 150, and 180 °C curing temperatures, respectively. As can be seen, the most favorable result obtained was 108° when the polymerization temperature reached 180 °C, while lower 89° and 99° were obtained at milder curing temperatures of 150 °C and 125 °C, respectively. Besides the above investigation, with sample CT5, we also explored the possibility of achieving acceptable results by reducing the curing temperature further to 110 °C but extending the curing time to 48 h. As reported in Table 4, although this treatment managed to shift the wettability toward less water-wet conditions, the contact angle remained in the range of 50–60°, suggesting that at this highly reduced temperature, the monomer had very likely attached to the rock surface through the silane moiety. Extensive polymerization had not occurred; therefore, surface energy could not be reduced to the necessary low levels. Given this unsuccessful outcome, the treatment conducted on CT5 was excluded from subsequent evaluations to evaluate the benzoxazine treatment’s performance at reduced curing temperatures. It is worth noting that the contact angle values reported in Table 4 are average values of 10 separate measurements conducted on a rock substrate at the experimental condition specified in the table.
Intermediate wettability conditions for the two-phase system are obtained when the water contact angle is in the range of 75 to 105°. (Anderson 1986; Wang et al. 2011b) Based on this classification, researchers investigating the application of fluorinated chemicals to alter rock wettability have typically reached values between 112° and 150° under similar conditions. (Al-Anazi et al. 2007; Fahes and Firoozabadi 2005; Franco-Aguirre et al. 2018; Li et al. 2011; Mousavi et al. 2013; Noh and Firoozabadi 2008; Zhang et al. 2014) In comparison, our results clearly demonstrate that the benzoxazine monomer can alter the surface of the rock from strongly water-wet to intermediate gas-wet conditions at even reduced polymerization temperatures during the treatment process. While the contact angle results were promising, as mentioned earlier, the spontaneous imbibition tests could be more insightful in revealing the performance of the benzoxazine treatment at reduced curing temperatures. The impure compounds in the synthesis process also affect the feasibility of polymerizing the benzoxazine resins based on the ring-opening process. Researchers evidenced that a non-catalytic polymerization in some benzoxazine types can occur even at 30 to 40 °C due to the phenolic impurities present in the synthesis process (Akkus et al. 2020; Han et al. 2017).
For a monomer concentration of 2.5 wt.%, Fig. 6 compares the pre- and post-treatment spontaneous brine imbibition results for samples CT2, CT3, and CT4 cured at 125, 150, and 180 °C, respectively. Overall, all three samples behaved similarly before treatment, reaching a plateau of about 65% spontaneously imbibed brine saturation at 16.7 h (Fig. 6). Although a similar data set looks more scattered for the three samples after treatment, the water uptake plateau decreased to an average value of about 55% at 16.7 h mark. These results suggest that the reduced 2.5 wt.% monomer concentration can still alter the rock wettability, producing an effect in the porous space to reduce the capillary strength while having only a tiny impact on the absolute permeability.
The contact angle data recorded for all curing temperatures explored (Table 4) do not follow the expected decreasing trend with a reduction in the polymerization temperature of the benzoxazine monomer as suggested elsewhere (Liu et al. 2013). A similar unconformity is evident among the spontaneous brine imbibition results reported in Fig. 6, where one may expect that a water-wet rock’s initial strength to imbibe water would be more preserved with a decrease in curing temperature. However, the above abnormal behavior may not seem highly unexpected considering the difficulties associated with achieving desirable outcomes when treating natural porous rocks with complex pore networks and internal pore surface systems as opposed to the treatments done on highly ideal and uniform surfaces as that of glass (Liu et al. 2013).
3.2 Core Flooding Results
To further understand the implication of the induced wettability alteration, core flooding experiments were conducted under elevated pressure and temperature before and after benzoxazine treatment on samples CT2, CT3 and CT4.
As a sample dataset, Fig. 7 compares the pre- and post-treatment differential pressure data (colored data points) recorded for the primary drainage floods conducted on CT3 during which gas displaced the initial brine-saturated sample. As can be seen, the treatment resulted in a reduction in the differential pressure data, which is more evident during post-breakthrough times. This reduction is attributed to improved gas relative permeability caused by enhanced brine removal from the sample’s pore space. These effects are desirable outcomes of the induced wettability alteration, and they agree with the results published by other researchers who treated rock samples with wettability-altering fluorinated chemicals (Aminnaji et al. 2015; Erfani Gahrooei and Ghazanfari 2017; Fahes and Firoozabadi 2005; Fahimpour et al. 2012; Jiang et al. 2013; Noh and Firoozabadi 2008). For example, Noh and Firoozabadi demonstrated a 24% reduction in differential pressure when applying a 4 wt.% concentration of a fluorinated chemical(Noh and Firoozabadi 2008).
The above outcome is taken as further evidence that benzoxazine treatment can alter the wettability of sandstone rocks from strongly water-wet to intermediate gas-wetting resulting in improved gas mobility by alleviating water blockage in low-permeability rocks. What is more, the above may be considered more significant than similar conclusions drawn from earlier discussed contact angle and spontaneous imbibition tests as the core flooding data (e.g., those reported in Fig. 7) were measured under fully dynamic fluid flow and elevated pressure and temperature conditions.
The brine recovery data recorded during primary drainage floods (Fig. 8 and Table 5) indicate that the benzoxazine treatment (across all curing temperatures explored) resulted in an average of 6.5% additional brine recovery. All these results align with the conclusions reported by Li et al. (2011) where they demonstrated that a fluorinated chemical could increase water recovery by around 15% during gas displacement in a sandstone rock. The observed enhancement in water recovery supports the earlier discussion presented around the shift in differential pressures (Fig. 7) and is seen as added evidence that our treatment approach can result in promising outcomes under dynamic fluid flow conditions. This increase in water recovery combined with the decrease in differential pressure agrees with the benzoxazine treatment’s wettability alteration mechanism we have proposed. The incremental brine recoveries obtained in this study are comparable with those reported in our previous study, in which we treated intermediate permeability samples with a much higher monomer concentration (10 wt.%) cured at 180 °C (Mauricio Ceron Lopez et al. 2021). This may demonstrate the outstanding effectiveness of the treatment in low-permeability rocks, as achieving enhanced recovery is more challenging under low-permeability conditions. Moreover, the additional brine recoveries obtained in the current work for either 125 or 150 °C curing temperatures are almost the same as that achieved for the optimal curing temperature of 180 °C (Fig. 8 and Table 5), demonstrating that this approach can be successfully applied under temperatures as low as 125 °C. In other words, although it is known that during polymerization, an increase in temperature enhances the strength of the intramolecular forces resulting in a further decrease in the surface free energy (i.e., higher level of wettability alteration), adequate results can still be obtained at the lower end of the temperature range.
To further elaborate on the alleviating effect that benzoxazine treatment can have on water blockage, Table 6 compares the end-point relative permeability values before and after treatment and the corresponding residual brine saturations for samples CT2, CT3 and CT4. As can be observed, CT4 (treated at 180 °C) exhibits a 20% improvement in the end-point relative permeability to gas and a 12% reduction in residual water saturation. While data reported for samples CT2 and CT3 reveal reductions in residual brine saturation (~ 11%) similar to that seen for CT4, their resultant enhancement in end-point gas relative permeability is similar (~ 7%) but considerably less than for CT4. Two factors occur during polymerization at elevated temperatures (ring opening and cross-linkage (Liu et al. 2013)), making this process simple and dependent on the curing conditions (temperature and time). The curing time remained constant to assess curing temperature, obtaining slightly better results on the CT4 in terms of end-point relative permeability and recovery factor. At the selected temperatures, the silane moiety creates a linkage between the benzoxazine monomer and the rock surface, reaching suitable chemical adsorption, while the ring opening of the benzoxazine monomer provides a reduced surface tension at values closer to 180 °C. At lower temperatures, the efficiency of the ring-opening process decreases during polymerization, leading to less favorable surface tension conditions of the treated rock sample (Liu et al. 2006, 2013).
The above results may not be as significant as those reported in the literature for fluorinated chemical treatments. However, the observed trends are in line with those seen for these chemicals (Li et al. 2011; Noh and Firoozabadi 2008). As elaborated in our previous work (Mauricio Ceron Lopez et al. 2021), it is also important to highlight that benzoxazine resin is significantly cheaper and less harmful to the environment than fluorinated compounds.
The earlier discussed shift in the post-treatment end-point gas relative permeability is evident across almost the entire relative permeability curves, as observed from the data in Fig. 9. This figure compares pre- and post-treatment relative permeability curves for the drainage floods conducted on sample CT3. Generally, the relative permeability to gas is higher after treatment with the monomer for fixed water saturation. The Sigmund and McCaffery set of equations (Eqs. 5, 6, 7) were used to perform the iterative process of history matching the pressure differential (Fig. 7—where DP_BT_SIM curve represents the differential pressure before treatment and the DP_AT_SIM represents the after treatment generated during the matching procedure) and brine recovery during the unsteady-state fluid displacement to finally determine the relative permeability of gas and brine based on the different procedures (Saeedi et al. 2016; Sigmund and McCaffery 1979). Compared with other models, such as Corey, the mentioned model seemed to capture better the complexity of the gas phase mobility, lower density, capillary-viscous ratio, and the shape of the relative permeability curves.
The values krw and krg are the relative permeability values of the wetting and non-wetting phase, respectively. Se is the normalized water saturation value, while krw' and krg' represent the relative permeability end-points of the wetting and non-wetting phases. The empirical constants Nw, Ng, A, and B were iteratively determined by different trials for history matching the differential pressure and displaced brine recovery. The Nw and Ng are values that minimize the error function between the observed and calculated pressure differential and displaced fluid recovery. In this process, the model also incorporates the capillary pressure effect into the relative permeability calculation, leading to a more suitable relative permeability calculation for the wetting and non-wetting phase that could be used in further analysis at the wellbore scale. Finally, the capillary pressure term has been obtained during the core-scale simulation modeling by the use of the commercial Sendra software by the use of Eq. 8 to, later on, use the corresponding capillary Eq. 9 to convert the capillary pressure values from laboratory conditions to a more real wellbore scale. These results were calculated before and after treatment to input the values in the wellbore model. The Cw, Co, aw, and ao are constant and non-negative parameters, where Cw, Co are expressed in units of pressure, while So represents the hydrocarbon phase saturation (Andersen et al. 2020).
Overall, the experimental procedure of water imbibition and flow displacement depicted moderate improvements in water recovery and relative permeability to gas compared with other researchers’ results using non-fluorinated and fluorinated chemicals. The flow displacement showed an increment of the end-point relative permeability values of 4 to 20%, while the residual water saturation decreased from 10 to 12% when treating the samples with a 2.5% chemical solution. The improvement mainly relies on the polymerization conditions of the chemical (curing temperature and time) and the purity of the raw chemical materials during the synthesis process of the benzoxazine resin. On the other hand, there are limited studies providing results associated with using non-fluorinated compounds to alter wettability; for example, a non-fluorinated analysis proposed by Arjomand, 2020 proposed a non-fluorinated silylation procedure at 60 °C to alter the wettability of a CO2-brine-rock system (Arjomand et al. 2020). This study showed that the chemical could increase 60% of the end-point CO2 relative permeability to obtain more than 25% water recovery. This procedure involved the use of CO2 as a solvent and carrier fluid. Our analysis focused on investigating an environmentally friendly chemical treatment that does not degrade or decrease its performance when increasing temperature; therefore, the hydrophobic characteristics increase, especially when reaching high polymerization temperatures such as 180 °C, which is the ideal curing temperature of the monomer.
On the other hand, extensive research has been conducted on applying fluorinated compounds to alter rock wettability. The results reveal that fluorinated compounds are the most effective alternative to alter wettability. For example, Noh, 2008 in his flow test analysis, obtained a 60 to 70% increment in water mobility using fluorinated chemicals and a drastic reduction of 90% approximately in the volume imbibed by the treated rock sample (Noh and Firoozabadi 2008). Those results surpass the effects of benzoxazine resin. When comparing the results of the benzoxazine resin with any of the fluorinated compounds, it is evident that the performance is not at the same level; the fluorinated compounds can lower the surface free energy of the rock considerably, reaching values lower than 5 mJ/m2 when applying fluorinated nano-silica components (Erfani Gahrooei and Ghazanfari 2017). Those results were obtained due to the characteristic of the chemical solution to modify the surface roughness and lower the surface free energy of the sample.
Additional analysis on the change of wettability from oil-wet to less oil-wet has been performed using the benzoxazine monomer to treat sandstone and carbonate rock samples. Therefore, the same procedure proposed in our analysis was performed for a gas-condensate-rock system using decane and hexadecane as the fluid phase. Additionally, a solution composed of benzoxazine and FAS was assessed to demonstrate that using a short-chain fluoropolymer is not enough to reach oleophobic results. When assessing the solutions proposed, the contact angle could not reach more than 5° in less than a minute during contact angle measurement. During spontaneous imbibition, the chemical solutions were unable to reduce the imbibition rate to any extent. Finally, the flow test analysis, using sandstone and carbonate rock samples, did not show any further improvement during water and gas displacement over the treated samples. Therefore, the surface free energy of the treated surface is not enough to repeal the liquid phase, as the surface tension of the liquids (decane and hexadecane) are 24 and 28 mJ/m2, respectively (Zisman 1964). In our previous research, we showed an approximated surface free energy of 34 mJ/m2, which is not enough to modify the wettability of the rock from oil-wet to less oil-wet (Mauricio Ceron Lopez et al. 2021). Based on the work done by Lui et al., glass surface with benzoxazine resin is treated to obtain less than 20 mJ/m2. This research demonstrates that oleophobic characteristics could alter wettability in glass surfaces (Liu et al. 2013). In our research, such conditions could not be achieved when treating glass and rock samples ranging from 10 to 130 mD. Curing time between 1 and 180 h and curing temperatures up to 180 °C were assessed to obtain lower surface free energy values; nevertheless, results did not depict promising results compared with those obtained by Liu. During the synthesis process, the phenolic impurities could probably affect the ring opening during thermal polymerization, leading to a drastic increment of the surface free energy value. Nevertheless, reaching such surface conditions is considerably low, benzoxazine can be used for water blockage applications.
3.3 Box Model Numerical Simulation Results
3.3.1 Effect of Invasion Radius Around the Wellbore
Figure 10 compares the pre- and post-treatment numerically simulated gas production profiles (over seven years) for a 15 mD rock formation assuming different radii of water invasion (4, 10, and 16 m). For further comparison, this figure also includes an additional curve representing the unaffected productivity of the same formation (i.e., no water invasion or ‘Initial Conditions’). As can be seen, the water invasion suppresses the gas productivity with a larger radius causing a more significant effect. The subsequent chemical treatment improves productivity across all cases explored, with its impact becoming more significant with an increase in the water invasion radius. The above comparison becomes more evident using the data included in Fig. 11, which compares the effects of water blockage on final simulated gas recovery for the above three water invasion radii and then reveals how much improvement the benzoxazine treatment can make across all explored cases. The figure shows that productivity loss ranges from 12.4 to 23.6% as the water invasion radius increases from 4 to 16 m, respectively. The chemical treatment then results in more remarkable incremental improvement for higher invasion radii increasing productivity between 2.9 and 10.6%.
Gas recovery profiles for various scenarios, including initial conditions (i.e., no water invasion), different radii of water invasion (4 (Case 1), 10 (Case 2), and 16 m (Case 3)), and after chemical treatment as generated using box model numerical simulation under an absolute permeability of 15 mD (BT: before treatment, AT: after treatment)
Percentage of recovery factor reduction due to water blockage at three cases of water invasion (4, 10, and 16 m) (blue color) and subsequent incremental improvement in recovery for the same invasion radii as caused by benzoxazine treatment (orange color) – Box model simulation under an absolute permeability of 15 mD
3.3.2 Effect of Absolute Rock Permeability
To evaluate the effect of absolute rock permeability on the performance of benzoxazine treatment as revealed by the box model simulations, we have used the relative permeability curves for CT2 from the current work and similar data generated for a BUG sample (~ 130 mD) from our previous study (Mauricio Ceron Lopez et al. 2021) in two separate simulation models. For this investigation, we have considered the intermediate water invasion radius of 10 m. As noted earlier, when the reservoir permeability is low, given the higher capillary strength, the reservoir is prone to a more pronounced water blockage effect.
The numerical simulation results in Fig. 12 show that, as indicated above, the negative effect of water blockage diminishes from 17.5% to a mere 0.6% when permeability increases from 15 to 130 mD. The same figure also indicates that upon treating the two rock types with benzoxazine, a recovery enhancement of 5.8% may be expected for the case of low-permeability rock compared with a negligible 0.1% effect for the case of higher permeability rock formation. This trend, resulting from the box model simulation, highlights that low-permeability formations are more prone to water blockage, presenting a higher potential for water blockage removal using the wettability alteration technique such as that developed in this research.
Percentage of recovery factor reduction due to water blockage at water invasion radius of 10 m for two absolute permeabilities of 15 and 130 mD (blue color) and subsequent incremental improvement in recovery for the same invasion radius and permeability values as caused by benzoxazine treatment (orange color) – results of box model numerical simulation
4 Summary and Conclusions
The effect of water blockage on gas deliverability is a significant concern in low-permeability reservoirs. Wettability alteration from a strong water-wet to an intermediate gas-wet condition using chemical treatments is a feasible remedial measure for this issue. The results reported in this manuscript complement those we reported in our previous study in showing benzoxazine resin to be a suitable alternative to previously tried fluorinated compounds in achieving the sought wettability shift under low-permeability conditions. Moreover, benzoxazine is an eco-friendly and cost-effective chemical compared with its fluorinated counterparts that exhibit environmental toxicity and tend to bio-accumulate as they do not readily biodegrade. The current results also demonstrate that our novel chemical treatment can alter wettability in low-permeability reservoirs at polymerization temperatures lower than the optimal value of 180 ºC, expanding the application envelope of the treatment to lower-temperature reservoirs. These effects were demonstrated by a combination of experimental analyses, which included contact angle measurements, spontaneous imbibition tests, and core-flooding experiments.
When treating a low-permeability sample with a concentration of 2.5 wt.% of the benzoxazine monomer, the absolute rock permeability decreases 3–10 wt.% of its initial value at different polymerization temperatures (125–180 °C). The spontaneous imbibition analysis demonstrated that the chemical could delay the water intake into the sample by decreasing the imbibition rate. Similarly, the contact angle after rock treatment increased from 0 to values between 89° and 108° at different polymerization temperatures. Therefore, the chemical solution can alter the surface free energy of the rock without drastically reducing the absolute gas permeability.
The core-flooding tests conducted at elevated pressure and temperature further validated the promising results of other experimental techniques. The results of these dynamics experiments indicated residual water saturation reduction of 10.8–12% after treatment when brine-saturated samples were flooded with gas. Furthermore, improvements in end-point gas relative permeability of 4–20% were also achieved. These results demonstrate that the benzoxazine resin could be potentially used to treat reservoirs with temperatures between 125 and 150 °C resulting in desirable outcomes.
The core-flooding tests conducted at elevated pressure and temperature further validated the promising results of other experimental techniques. The results of these dynamics experiments indicated residual water saturation reduction of 10.8–12% after treatment when brine-saturated samples were flooded with gas. Furthermore, improvements in end-point gas relative permeability of 4–20% were also achieved. These results demonstrate that the benzoxazine resin could be potentially used to treat reservoirs with temperatures between 125 and 150 °C resulting in desirable outcomes.
The application of the benzoxazine resin is primarily for reservoirs with high temperatures, such as deep gas reservoirs. The benzoxazine resin treatment can decrease the residual water saturation, while the gas relative permeability increases as well as water production. Those parameters are influenced by polymerization temperature during rock treatment, incrementing residual water saturation when the polymerization temperature increases, producing an improvement in relative permeability to gas. Those results demonstrate that the benzoxazine resin could be potentially used to treat reservoirs with temperatures between 125 and 150 °C.
Box model numerical simulations were used to upscale the results from core to wellbore scale. For this purpose, the simulation runs used the relative permeability data derived from core-flooding tests. The simulation results show that under low-permeability conditions (15 mD), productivity loss ranges from 12.4 to 23.6% as the water invasion radius increases from 4 to 16 m, respectively. The chemical treatment then resulted in greater incremental improvement for higher invasion radii increasing productivity between 2.9 and 10.6%. Our simulation results also indicated that low-permeability formations are more susceptible to water blockage, as demonstrated by gas recovery reductions of 17.5% to 0.6% obtained under 15 and 130 mD permeabilities (water invasion radius of 10 m), respectively. However, such formations are more suitable for water blockage removal using the wettability alteration technique such as the one developed in this research. For example, our simulation results indicate a 5.8% post-treatment increase in gas recovery under 15 mD formation permeability compared with a mere 0.1% increase under 130 mD.
Further to our previous work, the current coupled experimental and numerical study performed at two different scales (core vs. wellbore region) demonstrates the potential effectiveness of our novel chemical treatment in improving gas productivity under a wide range of application conditions.
The overall result of the benzoxazine monomer treatment to alter wettability in a gas–water-rock system is of great importance in achieving a non-fluorinated alternative to remediate water blockage; in this paper, a more realistic condition of permeability, chemical concentration, and polymerization temperature has been selected as the conditions to assess the chemical treatment. Moreover, a wellbore condition provided promising results in our trial to alter wettability. Further work could be done in synthesizing the monomer to improve thermal polymerization while maintaining or improving the surface free energy characteristics. It could lead to a more promising application to remove condensate blockage, which is considered challenging to alleviate without the implication of fluorinated compounds.
References
Akkus, B., Kiskan, B., Yagci, Y.: Cyanuric chloride as a potent catalyst for the reduction of curing temperature of benzoxazines. Polym. Chem. 11(5), 1025–1032 (2020)
Al-Anazi, H.A., Solares, J.R., Al-Faifi, M.: The impact of condensate blockage and completion fluids on gas productivity in gas-condensate reservoirs, SPE Asia Pacific Oil and Gas Conference and Exhibition. Society of Petroleum Engineers, Jakarta, Indonesia, pp. 11. (2005)
Al-Anazi, H.A., Xiao, J., Al-Eidan, A.A., Buhidma, I.M., Ahmed, M.S., Al-Faifi, M., Assiri, W.J.: Gas productivity enhancement by wettability alteration of gas-condensate reservoirs, european formation damage conference. Society of Petroleum Engineers, Scheveningen, The Netherlands, pp. 8. (2007)
Aminnaji, M., Fazeli, H., Bahramian, A., Gerami, S., Ghojavand, H.: Wettability alteration of reservoir rocks from liquid wetting to gas wetting using nanofluid. Transp. Porous Media 109(1), 201–216 (2015)
Andersen, P.Ø., Walrond, K., Nainggolan, C.K.L., Pulido, E.Y., Askarinezhad, R.: Simulation interpretation of capillary pressure and relative permeability from laboratory waterflooding experiments in preferentially oil-wet porous media. SPE Reservoir Eval. Eng. 23(01), 230–246 (2020)
Anderson, W.G.: Wettability literature survey- part 1: rock/oil/brine interactions and the effects of core handling on wettability. J. Petrol. Technol. 38(10), 1125–1144 (1986)
Arjomand, E., Myers, M., Al Hinai, N.M., Wood, C.D., Saeedi, A.: Modifying the wettability of sandstones using nonfluorinated silylation: to minimize the water blockage effect. Energy Fuels 34(1), 709–719 (2020)
Armenta, M., Wojtanowicz, A.: Severity of water coning in gas wells, SPE Gas Technology Symposium. Society of Petroleum Engineers, Calgary, Alberta, Canada, pp. 10. (2002)
Bahrami, H., Rezaee, M.R., Nazhat, D.H., Ostojic, J., Clennell, M.B., Jamili, A.: Effect of water blocking damage on flow efficiency and productivity in tight gas reservoirs, SPE Production and Operations Symposium. Society of Petroleum Engineers, Oklahoma City, Oklahoma, USA, pp. 7. (2011)
Bang, V.S.S., Pope, G.A., Sharma, M.M., Baran, J.R., Jr.: Development of a successful chemical treatment for gas wells with liquid blocking, SPE Annual Technical Conference and Exhibition. Society of Petroleum Engineers, New Orleans, Louisiana, pp. 10. (2009)
Bennion, D.B., Bietz, R.F., Thomas, F.B., Cimolai, M.P.: Reductions in the productivity of oil and low permeability gas reservoirs due to aqueous phase trapping. J. Can. Pet. Technol. 33(09), 11 (1994)
Bennion, D.B., Thomas, F.B., Bietz, R.F., Bennion, D.W.: Water And hydrocarbon phase trapping in porous media-diagnosis, prevention and treatment. J. Can. Pet. Technol. 35(10), 8 (1996c)
Bennion, B.D., Thomas, F.B., Bietz, R.F., Bennion, D.W.: Remediation of water and hydrocarbon phase trapping problems in low permeability gas reservoirs, Annual Technical Meeting. Petroleum Society of Canada, Calgary, Alberta, pp. 15. (1996a)
Bennion, D.B., Thomas, F.B., Bietz, R.F.: Low permeability gas reservoirs: problems, opportunities and solutions for drilling, completion, stimulation and production, SPE Gas Technology Symposium. Society of Petroleum Engineers, Calgary, Alberta, Canada, pp. 15. (1996b)
Bennion, D.B., Thomas, F.B., Ma, T.: Formation damage processes reducing productivity of low permeability gas reservoirs, spe rocky mountain regional/low-permeability reservoirs symposium and exhibition. Society of Petroleum Engineers, Denver, Colorado, pp. 19. (2000a)
Bennion, D.B., Thomas, F.B., Ma, T., 2000b. Recent Advances in Laboratory Test Protocols to Evaluate Optimum Drilling, Completion and Stimulation Practices for Low Permeability Gas Reservoirs.
Cimolai, M.P., Gies, R.M., Bennion, D.B., Myers, D.L.: Mitigating Horizontal Well Formation Damage in a Low-Permeability Conglomerate Gas Reservoir, SPE Gas Technology Symposium. Society of Petroleum Engineers, Calgary, Alberta, Canada, pp. 11. (1993)
Deng, L., King, M.J.: Theoretical Investigation of Water Blocking in Unconventional Reservoirs Due to Spontaneous Imbibition and Water Adsorption, SPE/AAPG/SEG Unconventional Resources Technology Conference. Unconventional Resources Technology Conference, Houston, Texas, USA, pp. 18. (2018)
Diana, L.: Investigations of Methods for Quantifying Diffusive Transport Processes in Sedimentary Rock, Nuclear Waste Management Organization, Toronto, Ontario. (2013)
Drummond, C., Israelachvili, J.: Surface forces and wettability. J. Petrol. Sci. Eng. 33(1), 123–133 (2002)
Eakin, J.L., Miller, J.S., Eckard, W.E. Removal of Water Blocks from Gas-producing Formations, Drilling and Production Practice. American Petroleum Institute, New York, New York, pp. 14. (1965)
Erfani Gahrooei, H.R., Ghazanfari, M.H.: Toward a hydrocarbon-based chemical for wettability alteration of reservoir rocks to gas wetting condition: Implications to gas condensate reservoirs. J. Mol. Liq. 248, 100–111 (2017)
Fahes, M.M., Firoozabadi, A.: Wettability Alteration to Intermediate Gas-Wetting in Gas-Condensate Reservoirs at High Temperatures, SPE Annual Technical Conference and Exhibition. Society of Petroleum Engineers, Dallas, Texas, pp. 14. (2005)
Fahimpour, J., Jamiolahmady, M., Sohrabi, M.: A Combined Experimental and Theoretical Investigation on Application of Wettability Modifiers in Gas-Condensate Reservoirs, SPE Annual Technical Conference and Exhibition. Society of Petroleum Engineers, San Antonio, Texas, USA, pp. 15. (2012)
Franco-Aguirre, M., Zabala, R.D., Lopera, S.H., Franco, C.A., Cortes, F.B.: Interaction of anionic surfactant-nanoparticles for gas - Wettability alteration of sandstone in tight gas-condensate reservoirs. J. Nat. Gas Sci. Eng. 51, 53–64 (2018)
Gogoi, N., Rastogi, D., Jassal, M., Agrawal, A.K.: Low-surface-energy materials based on polybenzoxazines for surface modification of textiles. J. Text. Inst. 105(11), 1212–1220 (2014)
Gupta, R., Mohanty, K.K.: Wettability alteration mechanism for oil recovery from fractured carbonate rocks. Transp. Porous Media 87(2), 635–652 (2011)
Han, L., Salum, M.L., Zhang, K., Froimowicz, P., Ishida, H.: Intrinsic self-initiating thermal ring-opening polymerization of 1,3-benzoxazines without the influence of impurities using very high purity crystals. J. Polym. Sci., Part a: Polym. Chem. 55(20), 3434–3445 (2017)
Holditch, S.A.: Factors affecting water blocking and gas flow from hydraulically fractured gas wells. J. Petrol. Technol. 31(12), 1515–1524 (1979)
IEA, I.E.A.: Sustainable_Recovery. In: IEA, I.E.A. (Editor). IEA, Paris (2020)
Jiang, G., Li, Y., Zhang, M.: Evaluation of gas wettability and its effects on fluid distribution and fluid flow in porous media. Pet. Sci. 10(4), 515–527 (2013)
Jin, J., Wang, Y., Wang, K., Ren, J., Bai, B., Dai, C.: The effect of fluorosurfactant-modified nano-silica on the gas-wetting alteration of sandstone in a CH4-liquid-core system. Fuel 178, 163–171 (2016)
Khan, Z.H., Noman, M., Hadi, A.: A Success Case of Water Blockage Treatment at Gas Well Through Alcohol Based Recipe, SPE/PAPG Pakistan Section Annual Technical Conference and Exhibition. Society of Petroleum Engineers, Islamabad, Pakistan, pp. 11. (2017)
Khansari, A.N. Evaluation of Well Productivity Loss Due to Formation Damage Caused by Spontaneous Imbibition in Underbalanced Drilling, IADC/SPE Managed Pressure Drilling and Underbalanced Operations Conference & Exhibition. Society of Petroleum Engineers, San Antonio, Texas, pp. 10. (2009)
Leckie, R.S.H.D.A.: Provenance of the lower cretaceous paddy member (Peace River Formation), northwestern Alberta. Bull. Can. Pet. Geol. 42, 482–498 (1994)
Li, K., Firoozabadi, A.: Experimental study of wettability alteration to preferential gas-wetting in porous media and its effects. SPE Reservoir Eval. Eng. 3(02), 139–149 (2000)
Li, K., Liu, Y., Zheng, H., Huang, G., Li, G.: Enhanced gas-condensate production by wettability alteration to gas wetness. J. Petrol. Sci. Eng. 78(2), 505–509 (2011)
Liao, C.-S., Wu, J.-S., Wang, C.-F., Chang, F.-C.: Modification of polymer substrates with low surface free energy material by low-temperature cured polybenzoxazine. Macromol. Rapid Commun. 29(1), 52–56 (2008)
Liu, Y., Zhang, W., Chen, Y., Zheng, S.: Polybenzoxazine containing polysilsesquioxane: Preparation and thermal properties. J. Appl. Polym. Sci. 99(3), 927–936 (2006)
Liu, J., Lu, X., Xin, Z., Zhou, C.: Synthesis and surface properties of low surface free energy silane-functional polybenzoxazine films. Langmuir 29(1), 411–416 (2013)
Lopez, M.C.G., Myers, M.B., Xie, Q., Wood, C.D., Saeedi, A.: Wettability alteration using benzoxazine resin: A remedy for water blockage in sandstone gas reservoirs. Fuel 291, 120189 (2021)
Mirzaei Paiaman, A., Moghadasi, J., Masihi, M.: Formation Damage Through Aqueous Phase Trapping in Gas Reservoirs, SPE Deep Gas Conference and Exhibition. Society of Petroleum Engineers, Manama, Bahrain, pp. 8. (2010)
Mousavi, M.A., Hassanajili, S., Rahimpour, M.R.: Synthesis of fluorinated nano-silica and its application in wettability alteration near-wellbore region in gas condensate reservoirs. Appl. Surf. Sci. 273, 205–214 (2013)
Noh, M.H., Firoozabadi, A.: Wettability alteration in gas-condensate reservoirs to mitigate well deliverability loss by water blocking. SPE Reservoir Eval. Eng. 11(04), 676–685 (2008)
Parekh, B., Sharma, M.M.: Cleanup of Water Blocks in Depleted Low-Permeability Reservoirs, SPE Annual Technical Conference and Exhibition. Society of Petroleum Engineers, Houston, Texas, pp. 12. (2004)
Park, S.-J., Seo, M.-K.: Chapter 3 - Solid-Liquid Interface. In: Park, S.-J., Seo, M.-K. (eds.) Interface Science and Technology, pp. 147–252. Elsevier (2011)
Park, J., Urata, C., Masheder, B., Cheng, D.F., Hozumi, A.: Long perfluoroalkyl chains are not required for dynamically oleophobic surfaces. Green Chem. 15(1), 100–104 (2013)
Rice, P.A.: C6-perfluorinated compounds: the new greaseproofing agents in food packaging. Curr. Environ. Health Rep. 2(1), 33–40 (2015)
Saeedi, A., Delle Piane, C., Esteban, L., Xie, Q.: Flood characteristic and fluid rock interactions of a supercritical CO2, brine, rock system: South West Hub, Western Australia. Int. J. Greenhouse Gas Control 54, 309–321 (2016)
Sigmund, P.M., McCaffery, F.G.: An improved unsteady-state procedure for determining the relative-permeability characteristics of heterogeneous porous media (includes associated papers 8028 and 8777). Soc. Petrol. Eng. J. 19(01), 15–28 (1979)
Wang, C.-F., Su, Y.-C., Kuo, S.-W., Huang, C.-F., Sheen, Y.-C., Chang, F.-C.: Low-Surface-Free-Energy Materials Based on Polybenzoxazines. Angew. Chem. Int. Ed. 45(14), 2248–2251 (2006)
Wang, Y., Xu, H., Yu, W., Bai, B., Song, X., Zhang, J.: Surfactant induced reservoir wettability alteration: Recent theoretical and experimental advances in enhanced oil recovery. Pet. Sci. 8(4), 463–476 (2011b)
Wang, C.-F., Chang, F.-C., Kuo, S.-W.: Surface Properties of Polybenzoxazines. Handbook of Benzoxazine Resins, pp. 579–593. (2011a)
Winkens, K., Vestergren, R., Berger, U., Cousins, I.T.: Early life exposure to per- and polyfluoroalkyl substances (PFASs): a critical review. Emerg. Contam. 3(2), 55–68 (2017)
Zhang, S., Jiang, G.-C., Wang, L., Qing, W., Guo, H.-T., Tang, X.-G., Bai, D.-G.: Wettability alteration to intermediate gas-wetting in low-permeability gas-condensate reservoirs. J. Pet. Explor. Prod. Technol. 4(3), 301–308 (2014)
Zisman, W.A.: Relation of the equilibrium contact angle to liquid and solid constitution, contact angle, wettability, and adhesion. Advances in Chemistry. American Chemical Society, pp. 1–51. (1964)
Acknowledgements
The author thanks Australian Technology Network Curtin South American Scholarship (ATN-LATAM) for providing economic support for this research. The authors also would like to thank Prores AS and Rock Flow Dynamics for providing their software’s academic license. The authors have no relevant financial or non-financial interests to disclose.
Funding
Open access funding provided by CSIRO Library Services. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lopez, G.M.C., Myers, M.B., Xie, Q. et al. Wettability Alteration to Reduce Water Blockage in Low-Permeability Sandstone Reservoirs. Transp Porous Med 147, 401–428 (2023). https://doi.org/10.1007/s11242-023-01914-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-023-01914-8