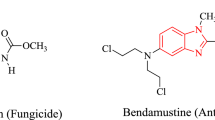
Abstract
Benzimidazoles/benzothiazoles are heterocyclic compounds which contain a five membered heteroatom and a benzene ring. They constitute a crucial structural unit of numerous bioactive compounds and natural products. Since the compounds containing benzimidazole/benzothiazole core and their derivatives possess interesting biological activity, steady efforts are being made on the development of an improved synthetic methodology for the synthesis of these biologically important class of compounds. Inspired by their biological properties, synthesis of 2-arylbenzimidazoles and 2-aryl benzothiazoles has been attempted using N^O chelate ruthenium(II)-catalyst in water. A series of 2-arylbenzimidazoles and 2-arylbenzothiazoles including a few new derivatives have been prepared by the reaction of ortho-phenylenediamine or ortho-aminothiophenol with aromatic aldehydes in the presence of 5 mol% of ruthenium(II)-catalyst under nitrogen without the use of additive in water. This reaction was extended to various heteroaromatic aldehydes obtaining up to 88% yield of the desired 2-arylbenzimidazoles/2-arylbenzothiazoles. In a few cases, a small amount of diarylated compounds was formed depending on the aldehydes used. Additionally, antibiotic properties of the synthesized compounds have been screened using the standard disc diffusion method.


Similar content being viewed by others
References
Noël S, Cadet S, Gras E, Hureau C (2013) Chem Soc Rev 42:7747–7762
Mann J, Baron A, Opoku-Boahen Y, Johansson E, Parkinson G, Kelland LR, Neidle S (2001) J Med Chem 44:138–144
Galal SA, Abdelsamie AS, Rodriguez ML, Kerwin SM, El Diwani HI (2010) Eur J Chem 2:67–72
Omar MA, Shaker YM, Galal SA, Ali MM, Kerwin SM, Li J, Tokuda H, Ramadan RA, El Diwani HI (2012) Bioorg Med Chem 20:6989–7001
Kumar BVS, Vaidya SD, Kumar RV, Bhirud SB, Mane RB (2006) Eur J Med Chem 41:599–604
Vinodkumar R, Vaidya SD, Kumar BVS, Bhise UN, Bhirud SB, Mashelkar UC (2008) Arkivoc 2008:37–49
Tonelli M, Simone M, Tasso B, Novelli F, Boido V, Sparatore F, Paglietti G, Pricl S, Giliberti G, Blois S (2010) Bioorg Med Chem 18:2937–2953
Goker H, Kus C, Boykin DW, Yildiz S, Altanlar N (2002) Bioorg Med Chem 10:2589–2596
Sharma D, Narasimhan B, Kumar P, Judge V, Narang R, Clercq ED, Balzarini J (2009) J Enzym Inhib Med Chem 24:1161–1168
Tolner B, Hartley JA, Hochhauser D (2001) Mol Pharmacol 59:699–706
Britten CD, Delioukina M, Boulos L, Reiswig L, Gicanov N, Rizzo J, Hao D, Tolcher A, Weitman S, Rugg T, Von Hoff D, Camden J, Rosen LS (2001) Proc Am Soc Clin Oncol 20:2129–2136
Duanmu C, Shahrik LK, Holly HH, Hamel E (1989) Cancer Res 49:134–138
Wright AE, Chiles SA, Cross SS (1991) J Nat Prod 54:1684–1686
Panda SS, Malik R, Jain SC (2012) Curr Org Chem 16:1905–1919
Sun Z, Bottari G, Barta K (2015) Green Chem 17:5172–5181
Alaqeel SI (2017) J Saudi Chem Soc 21:229–237
Kovvuri J, Nagaraju B, Kamal A, Srivastava AK (2016) ACS Comb Sci 18:644–650
Bahrami K, Khodaei MM, Nejati A (2010) Green Chem 12:1237–1241
Bahrami K, Khodaei MM, Naali F (2008) J Org Chem 73:6835–6837
Wang Y, Sarris K, Sauer DR, Djuric SWA (2006) Tetrahedron Lett 47:4823–4826
Maradolla MB, Allam SK, Mandha A, Chandramouli GVP (2008) Arkivoc 15:42–46
Dey M, Deb K, Dhar SS (2011) Chin Chem Lett 22:296–299
Renard G, Lerner DA (2007) New J Chem 31:1417–1420
Heravi MM, Sadjadi S, Oskooie HA, Shoar RH, Bamoharram FF (2007) Catal Commun 9:504–507
Keurulainen L, Salin O, Siiskonen A, Kern JM, Alvesalo J, Kiuru P, Maass M, Yli-Kauhaluoma J, Vuorela P (2010) J Med Chem 53:7664–7674
Hollan G, Samuel L, Ennis B, Hinde R (1967) J Chem Soc C 20–26.
Grimmett M R. (1984) Imidazoles and their benzo derivatives. In: Katritzky AR, Rees CW (eds) In Comprehensive heterocyclic chemistry, Pergamon, Oxford 5:457–487.
Preston P N (1984) Benzimidazoles and congeneric tricyclic compounds. In:Weissberger A, Taylor EC (eds) In the chemistry of heterocyclic compounds, Wiley, New York, Part 1, 40:6–60.
Lin SY, Isome Y, Stewart E, Liu JF, Yohannes D, Yu L (2006) Tetrahedron Lett 47:2883–2886
Nadaf RN, Siddiqui SA, Daniel T, Lahoti RJ, Srinivasan KV (2004) J Mol Catal A Chem 214:155–160
Kokare ND, Sangshetti JN, Shinde DB (2007) Synthesis 18:2829–2834
Yadav JS, Subba RBV, Premalatha K, Shiva SK (2008) Can J Chem 86:124–128
Varala R, Nasreen A, Enugala R, Adapa SR (2007) Tetrahedron Lett 48:69–72
Salehi P, Dabiri M, Zolfigol MA, Otokesh S, Baghbanzadeh M (2006) Tetrahedron Lett 47:2557–2560
Dudd LM, Venardou E, Garcia-Verdugo E, Licence P, Blake AJ, Wilson C, Poliakoff M (2003) Green Chem 5:187–192
Samanta S, Das S, Biswas P (2013) J Org Chem 78:11184–11193
Cao X, Cheng X, Bai Y, Liu S, Deng GJ (2014) Green Chem 16:4644–4648
Jui NT, Buchwald SL (2013) Angew Chem 125:11838–11841
Nguyen KMH, Largeron M (2015) Chem Eur J 21:12606–12610
Cao K, Tu YQ, Zhang FM (2010) Sci China Chem 53:130–134
Sharghi H, Beyzavi M H, Doroodmand M M (2008) Eur J Org Chem 4126–4138.
Saha P, Ramana T, Purkait N, Ali MA, Paul R, Punniyamurthy T (2009) J Org Chem 74:8719–8725
Chari MA, Sadanandam P, Shobha D, Mukkanti K (2010) J Heterocycl Chem 47:153–155
Kebede E, Tadikonda R, Nakka M, Inkollu B, Vidavalur S (2015) Eur J Org Chem 5929–5933.
Durgareddy GANK, Ravikumar R, Ravi S, Adapa SR (2013) J Chem Sci 125:175–182
Zhang LJ, Xia J, Zhou YQ, Wang H, Wang SW (2012) Synth Commun 42:328–336
Venkateswarlu Y, Kumar SR, Leelavathi P (2013) Org Med Chem Letts 3:7
Teruyuki K, Sungbong Y, Keun-Tae H, Masanobu K, Shinji K, Yoshihisa W (1991) Chem Lett 20:1275–1278
Cho CS, Kim JU (2008) Bull Korean Chem Soc 29:1097–1098
Khalafi-Nezhad A, Panahi F (2014) ACS Catal 4:1686–1692
Daw P, Ben-David Y, Milstein D (2017) ACS Catal 11:7456–7460
Singh KS, Devi P, Sawant SG, Kaminsky W (2015) Polyhedron 100:321–325
Bennett M A, Smith A K (1974) J Chem Soc Dalton Trans 233–241
Bennett MA, Huang TN, Matheson TW, Smith AK, Tucker PA (1980) Inorg Chem 19:1014–1021
Dayan O, Ozdemir N, Serbetci Z, Dincer M, Cetinkaya B, Buyukgungor O (2012) Inorg Chim Acta 392:246–253
Kirby W M M, Yoshihara G M, Sundsted K S, Warren J H (1957) Antibiot Annu 892–897.
Devi P, D’Souza L, Kamat T, Rodrigues C, Naik CG (2009) Indian J Mar Sci 38:38–44
Allegrucci M, Sauer K (2007) J Bacteriol 189:2030–2038
Dalton HM, Poulsen LK, Halasz P, Angles ML, Goodman AE, Marshall KC (1994) J Bacteriol 176:6900–6906
Banerjee M, Chatterjee A, Kumar V, Bhutia ZT, Khandare DG, Majik MS, Roy BG (2014) RSC Advances 4:39606–39611
Kumar V, Khandare DG, Chatterjee A, Banerjee M (2013) Tetrahedron Lett 54:5505–5509
Gopalaiah K, Chandrudu SN (2015) RSC Advances 5:5015–5023
Acknowledgements
We thank CSIR for financial support and Director, National Institute of Oceanography for providing facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, K.S., Joy, F. & Devi, P. Ruthenium(II)-catalyzed synthesis of 2-arylbenzimidazole and 2-arylbenzothiazole in water. Transit Met Chem 46, 181–190 (2021). https://doi.org/10.1007/s11243-020-00435-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-020-00435-3