Abstract
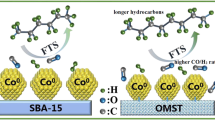
Iron oxide particles were deposited in an ordered mesoporous material (SBA-15) with the aim of studying its behavior in the catalytic hydrogenation of CO (Fischer–Tropsch Synthesis). Bulk iron oxide, and iron supported on porous silica with different textural properties (Aerosil®-200) were used for comparison. The characterization of the materials showed that in the Fe@SBA-15 material, iron nanoparticles were confined inside the mesopores of the SBA-15 support (pore diameter ~ 8 nm), and Fe@Aerosil®-200 material also presented iron oxide nanoparticles highly dispersed on the material. In situ Synchrotron radiation XRD studies were performed in order to study the evolution of iron phases in the Fe@SBA-15 and the bulk iron oxide under hydrogen and hydrogen/carbon monoxide conditions. DFT calculations were performed on bare Fe(100) and a Fe16 cluster in CO activation and CxHy hydrogenation. Catalytic microactivity tests, performed at conversions of ~ 6–8%, showed important differences in the selectivity of the materials. Higher selectivity to methane and light hydrocarbons were observed in the supported catalysts (Fe@SBA-15 and Fe@Aerosil®-200) than in bulk Fe catalyst. Moreover, the supported catalysts showed selectivity to ethylene (Fe@SBA-15) and propylene (Fe@Aerosil®-200), products that were not observed in the bulk iron catalyst. On the other hand, bulk iron showed a major selectivity to higher hydrocarbons (C5–C9) and oxygenates.








Similar content being viewed by others
References
Mesters C (2016) A selection of recent advances in C1 chemistry. Annu Rev Chem Biomol Eng 7:223–238
Abelló S, Montane D (2011) Exploring iron-based multifunctional catalysts for Fischer-Tropsch synthesis: a review. Chemsuschem 4:1538–1556
Cano LA, Cagnoli MV, Bengoa JF, Alvarez AM, Marchetti SG (2011) Effect of the activation atmosphere on the activity of Fe catalysts supported on SBA-15 in the Fischer-Tropsch Synthesis. J Catal 278:310–320
De Smit E, Cinquini F, Beale AM, Safonova OV, van Beek W, Sautet P, Weckhuysen BM (2010) Stability and reactivity of ϵ − χ−θ iron carbide catalyst phases in Fischer − Tropsch synthesis: controlling μC. J Am Chem Soc 132:14928–14941
Luo M, Hamdeh H, Davis BH (2009) Fischer-Tropsch synthesis: catalyst activation of low alpha iron catalyst. Catal Today 140:127–134
Cano LA, Garcia-Blanco AA, Lener G, Marchetti SG, Sapag K (2017) Effect of the support and promoters in Fischer-Tropsch synthesis using supported Fe catalysts. Catal Today 282:204–213
López C, Corma A (2012) Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Chem Cat Chem 4:751–752
Torres-Galvis HM, Bitter JH, Davindian T, Ruitenbeek M, Dugulan AI, de Jong KP (2012) Iron particle size effects for direct production of lower olefins from synthesis gas. J Am Chem Soc 134:16207–16215
Munnik P, de Jongh PE, de Jong KP (2015) Recent developments in the synthesis of supported catalysts. Chem Rev 115:6687–6718
Cheng K, Ordomsky VV, Virgine M, Legras B, Chernavskii PA, Kasak VO, Cordier C, Paul S, Wang Y, Khodakov AY (2014) Support effects in high temperature Fischer-Tropsch synthesis on iron catalysts. Appl Catal A 488:66–77
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chimelka BF, Stucky GD (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548–552
Barkhuizen D, Mabaso I, Viljoen E, Welker C, Claeys M, van Steen E, Fletcher JCQ (2006) Experimental approaches to the preparation of supported metal nanoparticles. Pure Appl Chem 78:1759–1769
Botes FG, Niemantsverdriet JW, van de Loosdrecht J (2013) A comparison of cobalt and iron based slurry phase Fischer-Tropsch synthesis. Catal Today 215:112–120
Park J-Y, Lee Y-J, Khanna PK, Jun K-W, Bae JW, Kim YH (2010) Alumina-supported iron oxide nanoparticles as Fischer-Tropsch catalysts: effect of particle size of iron oxide. J Mol Catal A 323:84–90
Abbaslou RMM, Soltan J, Dalai AK (2010) Effects of nanotubes pore size on the catalytic performances of iron catalysts supported on carbon nanotubes for Fischer-Tropsch synthesis. Appl Catal A 379:129–134
Cheng K, Virginie M, Ordomsky VV, Cordier C, Chernavskii PA, Ivantsov MI, Paul S, Wang Y, Khodakov AY (2015) Pore size effects in high-temperature Fischer-Tropsch synthesis over supported iron catalysts. J Catal 328:139–150
Hu Z, Ou Z, Yang L, Gao F, Xu B, Wu Q, Fan Y, Zhang Y, Jiang Y, Huang R, Wang X, Hu Z (2019) Stabilizing the active phase of iron-based Fischer-Tropsch catalysts for lower olefins: mechanism and strategy. Chem Sci 10:6015–6222
Chen W, Fan Z, Pan X, Bao X (2008) Effect of confinement in carbon nanotubes on the activity of Fischer − Tropsch iron catalyst. J Am Chem Soc 130:9414–9419
Gu B, Zhou C, He S, Moldovan S, Chernavskii PA, Ordomsky VV, Khodakov AY (2019) Size and promoter effects on iron nanoparticles confined in carbon nanotubes and their catalytic performance in light olefin synthesis from Syngas. Catal Today. https://doi.org/10.1016/j.cattod.2019.05.054
Zhao D, Hou Q, Feng J, Chmelka BF, Stucky GD (1998) Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J Am Chem Soc 120:6024–6036
Pérez-Alonso FJ (2006) Effect of the incorporation of ceramic oxide on the structure and reactivity of iron catalysts in the Fischer-Tropsch synthesis. PhD Thesis, Autonomous University of Madrid, Madrid, Spain
Villarroel-Rocha J, Barrera D, Sapag K (2014) Introducing a self-consistent test and the corresponding modification in the Barrett, Joyner and Halenda method for pore-size determination. Microporous Mesoporous Mater 200:68–78
Egerton RF (1996) Electron Energy Loss Spectroscopy in the Electron Microscope, 2nd edn. Plenum, New York
Zeng Y, Tan SJ, Do DD, Nicholson D (2016) Hysteresis and scanning curves in linear arrays of mesopores with two cavities and three necks. Colloids Surf A 496:52–62
Cychosz KA, Thommes M (2018) Progress in the physisorption characterization of nanoporous gas storage materials. Engineering 4:559–566
Technical Overview: Aerosil®—Fumed Silical (2007) Evonik, Esse. https://www.aerosil.com. Accessed 05 July 2019
Moreno MS, Weyland M, Midgley PA, Bengoa JF, Cagnoli MV, Gallegos NG, Marchetti SG (2006) Highly anisotropic distribution of iron nanoparticles within MCM-41 mesoporous silica. Micron 37:52–56
Sietsman JRA, Meeldijk JD, Versluijs-Helder M, Broersma A, Dillen AJ, van de Jongh PE, de Jong KP (2008) Ordered mesoporous silica to study the preparation of Ni/SiO2 ex nitrate catalysts: impregnation, drying, and thermal treatments. Chem Mater 20:2921–2931
Mos YM, Vermeulen AC, Buisman CNJ, Weijma J (2017) X-ray diffraction of iron containing samples: the importance of a suitable configuration. Solid State Phenom 262:545–548
Cheng H, Lu C, Liu J, Yan Y, Han X, Jin H, Wang Y, Liu Y, Wu C (2017) Synchrotron radiation X-ray powder diffraction techniques applied in hydrogen storage materials—a review. Prog Nat Sci-Matter 27:66–73
Mączka E, Kosmulski M (2015) Hematite and hematite–akageneite composites. XRD and electrokinetic study and interaction with ionic surfactants. J Colloid Interface Sci 458:130–135
Königer A, Hammerl C, Zeitler M, Rauschenbach B (1997) Formation of metastable iron carbide phases after high-fluence carbon ion implantation into iron at low temperatures. Phys Rev B 55:8143–8147
Raupp G, Delgass WN (1979) Mössbauer investigation of supported Fe and FeNi catalysts II. Carbides formed Fischer-Tropsch synthesis. J Catal 58:348–360
Schulz H, Schaub G, Claeys M, Riedel T (1999) Transient initial kinetic regimes of Fischer-Tropsch synthesis. Appl Catal A 186:215–227
Pérez-Alonso FJ, Herranz T, Rojas S, Ojeda M, López-Granados M, Terreros P, Fierro JLG, Gracia M, Gancedo JR (2007) Evolution of the bulk structure and surface species on Fe–Ce catalysts during the Fischer-Tropsch synthesis. Green Chem 9:663–670
Herranz T, Rojas S, Perez-Alonso F, Ojeda M, Terreros P, Fierro J (2006) Genesis of iron carbides and their role in the synthesis of hydrocarbons from synthesis gas. J Catal 243:199–211
Pérez-Alonso FJ, Ojeda M, Herranz T, González-Carballo JM, Fierro JLG, Bengoa JF, Marchetti SG (2008) Use of in situ Mössbauer spectroscopy and X-ray diffraction techniques for the characterization of activated Fe-Ce catalysts employed in Fischer-Tropsch synthesis. Open Magn Reson J 1:64–70
Hurst NW, Gentry SJ, McNicol BD (2007) Temperature programmed reduction. Catal Lett 24:233–309
Wei X, Zhou Y, Li Y, Shen W (2015) Polymorphous transformation of rod-shaped iron oxides and their catalytic properties in selective reduction of NO by NH3. RSC Adv 5:66141–66146
Kim SS, Lee SM, Hong SC (2012) A study on the reaction characteristics of CO2 decomposition using iron oxides. J Ind Eng Chem 18:860–864
Yogo K, Tanaka S, Ono T, Mikami T, Kikuchi E (1994) Characterization of Fe-silicates and their catalytic properties for the selective reduction of nitric oxide by hydrocarbons. Microporous Mater 3:39–46
Yiu HHP, Keane MA, Lethbridge ZAD, Lees MR, El Haj AJ, Dobson J (2008) Synthesis of novel magnetic iron metal–silica (Fe–SBA-15) and magnetite–silica (Fe3O4–SBA-15) nanocomposites with a high iron content using temperature-programed reduction. Nanotechnology 19:255606
Mosallanejad S, Dlugogorski BZ, Kennedy EM, Stockenhuber M (2018) On the chemistry of iron oxide supported on γ-alumina and silica catalysts. ACS Omega 3:5362–5374
Genz NS, Baabe D, Ressler T (2017) Solid-state kinetic investigations of nonisothermal reduction of iron species supported on SBA-15. J Anal Methods Chem 2017:1–13
Zhibin L, Shiying B, Liqin D, Haian X, Qian X, Yindi C, Lizhen A, Mingyi Z, An-Ya L, Shang-Bin L (2009) Fe2O3/SBA-15 catalyst synthesized by chemical vapor infiltration for Friedel-Crafts alkylation reaction. Microporous Mesoporous Mater 123:306–313
Hong SY, Chun DH, Yang JI, Jung H, Lee HT, Hong S, Jang S, Lim JT, Kim CS, Park JC (2015) A new synthesis of carbon encapsulated Fe5C2 nanoparticles for high-temperature Fischer-Tropsch synthesis. Nanoscale 7:16616–16620
Otero GS, Pascucci B, Branda MM, Belelli PG (2016) Evaluating the size of Fe nanoparticles for ammonia adsorption and dehydrogenation. Comput Mater Sci 124:220
Acknowledgements
The authors wish to thank to UNSL and CONICET for the financial support of this work. The authors acknowledge Brazilian Synchrotron Light Laboratory (LNLS) for the use of XPD beam line and for partial financial support (under proposal 20171027).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Toncón-Leal, C.F., Amaya-Roncancio, S., García Blanco, A.A. et al. Confined Iron Nanoparticles on Mesoporous Ordered Silica for Fischer–Tropsch Synthesis. Top Catal 62, 1086–1095 (2019). https://doi.org/10.1007/s11244-019-01201-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-019-01201-1