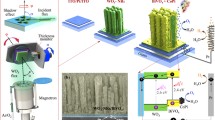
Abstract
We report the microwave-assisted synthesis of heterojunctions based on bismuth niobate (BiNbO4) and tungsten oxide (WO3). These architectures have been used as photoanodes for water splitting under simulated AM1.5G solar light. We show for the first time that by controlling temperature and irradiation power it is possible to tune the fraction of orthorhombic and triclinic phases in BiNbO4 nanoparticles, with strong consequences on the photocatalytic capabilities of the resulting heterojunctions. XRD patterns show that lower temperatures and irradiation power favor the formation of triclinic BiNbO4 arrangements, whereas the morphology of WO3 films is straightforwardly controlled by the addition of weak acids in the reactional medium that enable the formation of wrinkle-rod or cube-like particles. The orthorhombic symmetry of BiNbO4 is shown to decrease the bandgap energy, whereas wrinkle-rod nanoparticles of WO3 provides a rough surface that enhances the interaction between the semiconductors. This strategy leads to heterojunction able to generate photocurrent densities more than one order of magnitude higher than of bare WO3 film. Our findings demonstrate that the microwave-assisted route is a very attractive alternative to directly control crystalline structure and ultimately the photocatalytic performance of bismuth niobate- and tungsten oxide-based heterojunctions.







Similar content being viewed by others
References
Jain IP (2009) Hydrogen the fuel for 21st century. Int J Hydrogen Energy 34(17):7368–7378. https://doi.org/10.1016/j.ijhydene.2009.05.093
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358):37–38
Aroutiounian VM, Arakelyan VM, Shahnazaryan GE (2005) Metal oxide photoelectrodes for hydrogen generation using solar radiation-driven water splitting. Sol Energy 78(5):581–592. https://doi.org/10.1016/j.solener.2004.02.002
Yang Y, Niu SW, Han DD, Liu TY, Wang GM, Li Y (2017) Progress in developing metal oxide nanomaterials for photoelectrochemical water splitting. Adv Energy Mater. https://doi.org/10.1002/aenm.201700555
Maeda K, Domen K (2010) Photocatalytic water splitting: recent progress and future challenges. J Phys Chem Lett 1(18):2655–2661. https://doi.org/10.1021/jz1007966
Bavykin DV, Walsh FC (2010) Titanate and titania nanotubes: synthesis, properties and applications. Royal Society of Chemistry, London
Pan H (2016) Principles on design and fabrication of nanomaterials as photocatalysts for water-splitting. Renew Sustain Energy Rev 57(Supplement C):584–601. https://doi.org/10.1016/j.rser.2015.12.117
Chen S, Thind SS, Chen A (2016) Nanostructured materials for water splitting—state of the art and future needs: a mini-review. Electrochem Commun 63(Supplement C):10–17. https://doi.org/10.1016/j.elecom.2015.12.003
Ismail AA, Bahnemann DW (2014) Photochemical splitting of water for hydrogen production by photocatalysis: a review. Solar Energy Mater Solar Cells 128(Supplement C):85–101. https://doi.org/10.1016/j.solmat.2014.04.037
Osterloh FE (2013) Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem Soc Rev 42(6):2294–2320. https://doi.org/10.1039/c2cs35266d
Weng BC, Grice CR, Ge J, Poudel T, Deng XM, Yan YF (2018) Barium bismuth niobate double perovskite/tungsten oxide nanosheet photoanode for high-performance photoelectrochemical water splitting. Adv Energy Mater. https://doi.org/10.1002/aenm.201701655
Kulkarni AK, Sethi YA, Panmand RP, Nikam LK, Baeg J-O, Munirathnam NR, Ghule AV, Kale BB (2017) Mesoporous cadmium bismuth niobate (CdBi2Nb2O9) nanospheres for hydrogen generation under visible light. J Energy Chem 26(3):433–439. https://doi.org/10.1016/j.jechem.2016.12.012
Depablos-Rivera O, Medina JC, Bizarro M, Martínez A, Zeinert A, Rodil SE (2017) Synthesis and properties of Bi5Nb3O15 thin films prepared by dual co-sputtering. J Alloys Compd 695:3704–3713. https://doi.org/10.1016/j.jallcom.2016.11.340
Gurunathan K, Maruthamuthu P (1998) Bi5Nb3O15 as a photocatalyst: photocatalytic and photoelectrochemical studies. J Solid State Electrochem 2(3):176–180. https://doi.org/10.1007/s100080050084
Almeida CG, Araujo RB, Yoshimura RG, Mascarenhas AJ, da Silva AF, Araujo CM, Silva LA (2014) Photocatalytic hydrogen production with visible light over Mo and Cr-doped BiNb(Ta)O4. Int J Hydrogen Energy 39(3):1220–1227
Depablos-Rivera O, Zeinert A, Rodil SE (2018) Synthesis and optical properties of different bismuth niobate films grown by dual magnetron co-sputtering. Adv Eng Mater. https://doi.org/10.1002/adem.201800269
Xing Z, Wang ZG, Huang WB (2019) Enhanced room-temperature microwave dielectric properties in bismuth zinc niobate thin films. J Alloys Compd 798:665–668. https://doi.org/10.1016/j.jallcom.2019.05.315
Devesa S, Graca MP, Henry F, Costa LC (2015) Microwave dielectric properties of (Bi1-xFex)NbO4 ceramics prepared by the sol gel method. Ceram Int 41(6):8186–8190. https://doi.org/10.1016/j.ceramint.2015.03.038
Gao LB, Jiang SW, Li RG, Li B, Li YR (2014) Effect of magnesium content on structure and dielectric properties of cubic bismuth magnesium niobate pyrochlores. Ceram Int 40(3):4225–4229. https://doi.org/10.1016/j.ceramint.2013.08.085
Wang Z, Ren W, Zhan XL, Shi P, Wu XQ (2014) Structure, composition and microwave dielectric properties of bismuth zinc niobate pyrochlore thin films. J Appl Phys 116(19):194107. https://doi.org/10.1063/1.4902172
Zhang S, Yang Y, Guo Y, Guo W, Wang M, Guo Y, Huo M (2013) Preparation and enhanced visible-light photocatalytic activity of graphitic carbon nitride/bismuth niobate heterojunctions. J Hazard Mater 261:235–245. https://doi.org/10.1016/j.jhazmat.2013.07.025
Gan HH, Zhang GK, Guo YD (2012) Facile in situ synthesis of the bismuth oxychloride/bismuth niobate/TiO2 composite as a high efficient and stable visible light driven photocatalyst. J Colloid Interface Sci 386:373–380. https://doi.org/10.1016/j.jcis.2012.07.014
Min YL, Zhang FJ, Zhao W, Zheng FC, Chen YC, Zhang YG (2012) Hydrothermal synthesis of nanosized bismuth niobate and enhanced photocatalytic activity by coupling of graphene sheets. Chem Eng J 209:215–222. https://doi.org/10.1016/j.cej.2012.07.109
Gan HH, Zhang GK, Huang HX (2013) Enhanced visible-light-driven photocatalytic inactivation of Escherichia coli by Bi2O2CO3/Bi3NbO7 composites. J Hazard Mater 250:131–137. https://doi.org/10.1016/j.jhazmat.2013.01.066
Muktha B, Darriet J, Madras G, Guru Row TN (2006) Crystal structures and photocatalysis of the triclinic polymorphs of BiNbO4 and BiTaO4. J Solid State Chem 179(12):3919–3925. https://doi.org/10.1016/j.jssc.2006.08.032
Lee C-Y, Macquart R, Zhou Q, Kennedy BJ (2003) Structural and spectroscopic studies of BiTa1−xNbxO4. J Solid State Chem 174(2):310–318. https://doi.org/10.1016/S0022-4596(03)00225-1
Fajrina N, Tahir M (2019) A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int J Hydrogen Energy 44(2):540–577. https://doi.org/10.1016/j.ijhydene.2018.10.200
Faraji M, Yousefi M, Yousefzadeh S, Zirak M, Naseri N, Jeon TH, Choi W, Moshfegh AZ (2019) Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ Sci 12(1):59–95. https://doi.org/10.1039/c8ee00886h
Hisatomi T, Domen K (2019) Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat Catal 2(5):387–399. https://doi.org/10.1038/s41929-019-0242-6
Afroz K, Moniruddin M, Bakranov N, Kudaibergenov S, Nuraje N (2018) A heterojunction strategy to improve the visible light sensitive water splitting performance of photocatalytic materials. J Mater Chem A 6(44):21696–21718. https://doi.org/10.1039/c8ta04165b
Claudino CH, Kuznetsova M, Rodrigues BS, Chen C, Wang Z, Sardela M, Souza JS (2020) Facile one-pot microwave-assisted synthesis of tungsten-doped BiVO4/WO3 heterojunctions with enhanced photocatalytic activity. Mater Res Bull 125:110783. https://doi.org/10.1016/j.materresbull.2020.110783
Souza JS, Carvalho WM, Souza FL, Ponce-de-Leon C, Bavykin DV, Alves WA (2016) Multihierarchical electrodes based on titanate nanotubes and zinc oxide nanorods for photoelectrochemical water splitting. J Mater Chem A 4(3):944–952. https://doi.org/10.1039/c5ta06646h
de Almeida RM, Ferrari VC, dos S. Souza J, Souza FL, Alves WA, (2020) Tailoring a zinc oxide nanorod surface by adding an earth-abundant cocatalyst for induced sunlight water oxidation. ChemPhysChem 21(6):476–483. https://doi.org/10.1002/cphc.201901171
Kim JK, Moon JH, Lee T-W, Park JH (2012) Inverse opal tungsten trioxide films with mesoporous skeletons: synthesis and photoelectrochemical responses. Chem Commun 48(98):11939–11941. https://doi.org/10.1039/c2cc36984b
Zhu T, Chong MN, Chan ES (2014) Nanostructured tungsten trioxide thin films synthesized for photoelectrocatalytic water oxidation: a review. Chemsuschem 7(11):2974–2997. https://doi.org/10.1002/cssc.201402089
Waller MR, Townsend TK, Zhao J, Sabio EM, Chamousis RL, Browning ND, Osterloh FE (2012) Single-crystal tungsten oxide nanosheets: photochemical water oxidation in the quantum confinement regime. Chem Mater 24(4):698–704. https://doi.org/10.1021/cm203293j
Reinhard S, Rechberger F, Niederberger M (2016) Commercially available WO3 nanopowders for photoelectrochemical water splitting: photocurrent versus oxygen evolution. ChemPlusChem 81(9):935–940. https://doi.org/10.1002/cplu.201600241
Bilecka I, Niederberger M (2010) Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2(8):1358–1374. https://doi.org/10.1039/b9nr00377k
Brittany L, Hayes PD (2002) Microwave synthesis chemistry at the speed of light. CEM Publishing, Matthews
Nuchter M, Ondruschka B, Bonrath W, Gum A (2004) Microwave assisted synthesis—a critical technology overview. Green Chem 6(3):128–141. https://doi.org/10.1039/b310502d
Tsuji M, Hashimoto M, Nishizawa Y, Kubokawa M, Tsuji T (2005) Microwave-assisted synthesis of metallic nanostructures in solution. Chem Eur J 11(2):440–452. https://doi.org/10.1002/chem.200400417
Zhu Y-J, Chen F (2014) Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem Rev 114(12):6462–6555. https://doi.org/10.1021/cr400366s
Hariharan V, Gnanavel B, Aroulmoji V, Prabakaran K (2019) Role of chromium in tungsten oxide (WO3) by microwave irradiation technique for sensor applications. Indian J Phys 93(4):459–465. https://doi.org/10.1007/s12648-018-1310-5
Palanisamy P, Thangavel K, Murugesan S, Marappan S, Chavali M, Siril PF, Perumal DV (2019) Investigating the synergistic effect of hybridized WO3-ZnS nanocomposite prepared by microwave-assisted wet chemical method for supercapacitor application. J Electroanal Chem 833:93–104. https://doi.org/10.1016/j.jelechem.2018.11.026
Durairaj A, Jennifer DL, Sakthivel T, Obadiah A, Vasanthkumar S (2018) Development of tungsten disulfide ZnO nanohybrid photocatalyst for organic pollutants removal. J Mater Sci Mater Electron 29(22):19413–19424. https://doi.org/10.1007/s10854-018-0070-5
Movlaee K, Periasamy P, Krishnakumar T, Ganjali MR, Leonardi SG, Neri G, Chavali M, Siril PF, Devarajan VP (2018) Microwave-assisted synthesis and characterization of WOx nanostructures for gas sensor application. J Alloys Compd 762:745–753. https://doi.org/10.1016/j.jallcom.2018.05.189
Nagaraju P, Alsalme A, Alkathiri AM, Jayavel R (2018) Rapid synthesis of WO3/graphene nanocomposite via in-situ microwave method with improved electrochemical properties. J Phys Chem Solids 120:250–260. https://doi.org/10.1016/j.jpcs.2018.04.046
Periasamy P, Krishnakumar T, Sathish M, Devarajan VP, Siril PF, Chavali M (2018) Investigation of electrochemical properties of microwave irradiated tungsten oxide (WO3) nanorod structures for supercapacitor electrode in KOH electrolyte. Mater Res Express 5(8):085007. https://doi.org/10.1088/2053-1591/aad1dc
Simoes AZ, Ramirez MA, Ries A, Wang F, Longo E, Varela JA (2006) Microwave synthesis of calcium bismuth niobate thin films obtained by the polymeric precursor method. Mater Res Bull 41(8):1461–1467. https://doi.org/10.1016/j.materresbull.2006.01.025
Singh R, Luthra V, Tandon RP (2016) Microwave-assisted sintering of non-stoichiometric strontium bismuth niobate ceramic: structural and dielectric properties. Physica B 500:169–178. https://doi.org/10.1016/j.physb.2016.08.003
Goncalves LF, Rocha LSR, Silva CC, Cortés JA, Ramirez MA, Simões AZ (2016) Dielectric properties of bismuth niobate films using LaNiO3 bottom electrode. J Mater Sci Mater Electron 27(3):2866–2874. https://doi.org/10.1007/s10854-015-4103-z
Stoltzfus MW, Woodward PM, Seshadri R, Klepeis J-H, Bursten B (2007) Structure and Bonding in SnWO4, PbWO4, and BiVO4: lone pairs vs inert pairs. Inorg Chem 46(10):3839–3850. https://doi.org/10.1021/ic061157g
Hong SJ, Lee S, Jang JS, Lee JS (2011) Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ Sci 4(5):1781–1787. https://doi.org/10.1039/c0ee00743a
Litimein F, Khenata R, Gupta SK, Murtaza G, Reshak AH, Bouhemadou A, Bin Omran S, Yousaf M, Jha PK (2014) Structural, electronic, and optical properties of orthorhombic and triclinic BiNbO4 determined via DFT calculations. J Mater Sci 49(22):7809–7818. https://doi.org/10.1007/s10853-014-8491-x
Li L, Zhao J, Wang Y, Li Y, Ma D, Zhao Y, Hou S, Hao X (2011) Oxalic acid mediated synthesis of WO3·H2O nanoplates and self-assembled nanoflowers under mild conditions. J Solid State Chem 184(7):1661–1665. https://doi.org/10.1016/j.jssc.2011.05.008
Salje E (1977) The orthorhombic phase of WO3. Acta Crystallogr B 33(2):574–577. https://doi.org/10.1107/S0567740877004130
Deb SK (1973) Optical and photoelectric properties and colour centres in thin films of tungsten oxide. Philos Mag 27(4):801–822. https://doi.org/10.1080/14786437308227562
González-Borrero PP, Sato F, Medina AN, Baesso ML, Bento AC, Baldissera G, Persson C, Niklasson GA, Granqvist CG, Silva AFD (2010) Optical band-gap determination of nanostructured WO3 film. Appl Phys Lett 96(6):061909. https://doi.org/10.1063/1.3313945
Di Valentin C, Wang F, Pacchioni G (2013) Tungsten oxide in catalysis and photocatalysis: hints from DFT. Top Catal 56(15):1404–1419. https://doi.org/10.1007/s11244-013-0147-6
Acknowledgements
This work was supported by FAPESP (Grants 2017/11395-7 and 2017/26633-0) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The authors also acknowledge Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We are thankful to LNNano-CNPEM for the use of TEM and SEM facilities, to LNLS-CNPEM for the XRD experiments and to the Multiusers platform (CEM) at UFABC for instrumental facilities.
Author information
Authors and Affiliations
Contributions
JSS designed and supervised the research. MK and SAAO performed the synthesis and characterization of BiNbO4 and analyzed the data. MK and BSR performed the synthesis and characterization of WO3 films and analyzed the data. MK performed the electrochemical characterization. JSS has written the manuscript based on inputs from all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kuznetsova, M., Oliveira, S.A.A., Rodrigues, B.S. et al. Microwave-Assisted Synthesis of Bismuth Niobate/Tungsten Oxide Photoanodes for Water Splitting. Top Catal 64, 748–757 (2021). https://doi.org/10.1007/s11244-020-01325-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01325-9