Abstract
Extracellular ATP and 5-hydroxytryptamine (5-HT) are both involved in visceral sensory pathways by interacting with P2X and 5-HT3 receptors, respectively. We have investigated the changes in P2X and 5-HT3-mediated signalling in pelvic afferent neurons in mice deficient in P2X2 and/or P2X3 subunits by whole-cell recording of L6–S2 dorsal root ganglion (DRG) neurons and by multi-unit recording of pelvic afferents of the colorectum. In wildtype DRG neurons, ATP evoked transient, sustained or mixed (biphasic) inward currents. Transient currents were absent in P2X3 −/− neurons, whereas sustained currents were absent in P2X2 −/− DRG neurons. Neither transient nor sustained currents were observed following application of ATP or α,β-methylene ATP (α,β-meATP) in P2X2/P2X3 Dbl−/− DRG neurons. 5-HT was found to induce a fast inward current in 63% of DRG neurons from wildtype mice, which was blocked by tropisetron, a 5-HT3 receptor antagonist. The percentage of DRG neurons responding to 5-HT was significantly increased in P2X 2 −/−, P2X3 −/− and P2X2/P2X3 Dbl−/− mice, and the amplitude of 5-HT response was significantly increased in P2X2/P2X3 Dbl−/− mice. The pelvic afferent response to colorectal distension was attenuated in P2X2/P2X3 Dbl−/− mice, but the response to serosal application of 5-HT was enhanced. Furthermore, tropisetron resulted in a greater reduction in pelvic afferent responses to colorectal distension in the P2X2/P2X3 Dbl−/− preparations. These data suggest that P2X receptors containing the P2X2 and/or P2X3 subunits mediate purinergic activation of colorectal afferents and that 5-HT signalling in pelvic afferent neurons is up-regulated in mice lacking P2X2 or P2X3 receptor genes. This effect is more pronounced when both subunits are absent.
Similar content being viewed by others
Introduction
Extracellular ATP has been implicated in visceral sensory pathways as well as in somatosensory signal transduction. ATP evokes rapid excitation of mesenteric afferent fibres supplying the small intestine in the rat [1], pelvic afferent fibres of the rat colorectum [2] and the mouse urinary bladder [3, 4]. Pelvic afferent sensitivity to distension of the urinary bladder in the mouse and the colorectum in the rat could be attenuated by P2 purinoceptor antagonists. It has been proposed that the underlying mechanism is a purinergic mechanosensory transduction, where mechanical stimulation of tube and sac organs results in the release of ATP from epithelial cells lining these organs, and this ATP, in turn, stimulates afferent terminals in the subepithelial layers [5]. It is generally believed that the actions of ATP on afferent neurons are through interactions with P2X receptors containing the P2X2 and/or P2X3 subunits [6, 7]. While homomeric P2X3 receptors give rise to rapidly desensitising responses and are activated by α,β-methylene ATP (α,β-meATP), P2X2 receptors give a sustained response to ATP but are insensitive to α,β-meATP. Heteromeric P2X2/3 receptors give a slowly desensitising response to both agonists. However, pharmacological tools for studying P2X receptor mechanisms are still very limited. The development of P2X2 and/or P2X3 null-mutant mice has enabled us to investigate the effects of selective loss of these receptor subunits and, thus, the role of P2X receptors in visceral sensation. P2X3 knockout studies have revealed an important role of purinergic signalling in sensory control of the urinary bladder [3, 8]. However, while some changes in peristaltic activity were observed in vitro, gastrointestinal transit in these animals was little changed [9].
5-Hydroxytryptamine (5-HT) is also known to be an important mediator of visceral afferent activation. 5-HT can rapidly stimulate mesenteric afferent fibres of the small intestine as well as the colonic afferents [10]. Multiple subtypes of serotonin receptors are expressed in sensory neurons. However, it appears that the rapid actions of 5-HT are mediated by 5-HT3 receptors [11–13], which are part of the nicotinic receptor superfamily of ligand-gated ion channels. 5-HT3 and P2X receptors are both expressed in dorsal root ganglia (DRG) neurons [14–18]. There is evidence that 5-HT3 and P2X receptors may interact in a mutual inhibitory manner [19, 20], although the functional significance of this interaction remains unclear.
It has been recognised that deletion of a receptor gene can result in adaptive compensatory changes in related signalling pathways during development [21]. Thus, loss of purinergic afferent signalling may be replaced by upregulation of other systems. In the current study, we aimed to determine the possible changes in 5-HT3-mediated signalling in the pelvic afferent neurons from mice with targeted deletion of genes encoding P2X2 and/or P2X3 subunits. We performed whole-cell recordings of dispersed DRG cells from the L6-S2 segments, which include the primary afferent neurons innervating the colon, and compared the effects of 5-HT in cells from P2X2 and/or P2X3 deficient mice and their wildtype controls. We also investigated changes in pelvic afferent sensitivity to colorectal distension and 5-HT in an in vitro colorectal preparations using P2X2/P2X3 Dbl−/− mice and their wildtype controls. The results suggest an adaptive increase in 5-HT3-mediated signalling in pelvic afferent neurons in mice deficient in P2X2 and/or P2X3 subunits.
Materials and methods
Animals
P2X3 −/− mice were generated as described previously in detail [8]. P2X2 −/− mice were generated by the introduction of a deletion encompassing exons 2–11 and replacement of this sequence with a LoxP-flanked neomycin resistance gene into the mouse P2X2 gene (see [22]). P2X2/P2X3 Dbl−/− mice were generated by the crossing of 129Ola × C57BL/6 P2X3 −/− F2 mice to 129Ola × C57BL/6J P2X2 −/− F2 mice to generate P2X2/P2X3 compound heterozygotes, which were bred to generate homozygous P2X2/P2X3 double wildtype and double knockout mice [22]. Although many P2X2/P2X3 Dbl−/− mice die before being weaned [23], those that survive go on to become outwardly healthy looking adults. Animals used in this study were 6 to 8 months old.
Whole-cell recording
Mice were killed by inhalation of a rising concentration of CO2, and death was confirmed by cardiac haemorrhage. DRGs were rapidly dissected out, desheathed and incubated in 4 ml Ca2+- and Mg2+-free Hanks’ balanced salt solution (HBSS) with 10 mM HEPES buffer (pH 7·4) (HBSS; Life Technologies) containing 1·5 mg ml−1 collagenase (class II, Worthington Biochemical Corporation, UK) and 6 mg ml−1 bovine serum albumin (Sigma Chemical Co., Poole, UK) at 37 °C for 45 min. The ganglia were then incubated in 4 ml HBSS containing 1 mg ml−1 trypsin (Sigma) at 37 °C for 15 min. The solution was replaced with 1 ml growth medium comprising L-15 medium supplemented with 10% bovine serum, 50 ng ml−1 nerve growth factor, 2 mg ml−1 NaHCO3, 5·5 mg ml−1 glucose, 200 i.u. ml−1 penicillin and 200 µg ml−1 streptomycin. The ganglia were then dissociated into single neurons by gentle trituration. The neurons were plated onto 35 mm Petri dishes coated with 10 µmg ml−1 laminin (Sigma) and maintained at 37 °C in a humidified atmosphere containing 5% CO2 and were used within 30 h.
Whole-cell voltage-clamp recordings were made from small and medium neurons having a diameter of less than 25 µmm which had a membrane capacitance of 39.8 ± 3.2 pF (n = 38). Experiments were carried out at room temperature using an Axopatch 200B amplifier (Axon Instruments, Foster City, Calif., USA) with membrane potential held at −60 mV. External solution contained (in millimoles): NaCl 154, KCl 4.7, MgCl2 1.2, CaCl2 2.5, HEPES 10 and glucose 5.6; the pH was adjusted to 7.4 with NaOH. Recording electrodes (resistance 2–4 MΩ) were filled with internal solution that contained (in millimoles): KCl 120, HEPES 10, tri-potassium citrate 10; EGTA 10. The pH was adjusted to 7·2 with KOH. Current signals were acquired with pClamp software (version 6.1, Axon Instruments) and were plotted using Origin7 (Microcal, Northampton, Mass., USA).
Drugs were applied rapidly through a manifold comprising seven capillaries made of fused silica coated with polyimide, with 250 µm internal diameter (SGE, Milton Keynes, UK), connected to a single outlet made of the same tubing, which was placed approximately 200 µm from the cell. Solutions were delivered by gravity flow from independent reservoirs. One barrel was used to apply drug-free solution to enable rapid termination of drug applications. Solution exchange measured by changes in open tip current was complete in 200 ms; however, complete exchange of solution around an intact cell was considerably slower (1 s). Nevertheless, solution exchange using this system was fast enough for us to observe rapidly desensitising responses in DRG neurons (see [24]). Agonists were separately applied for 2 s at 4 min intervals, times which were sufficient for responses to be reproducible. Antagonists were pre-applied for 2 min and then coapplied with agonists.
Recording of pelvic afferent activity in an in vitro colorectal preparation
The distal colon and rectum were dissected from the pelvis with attached pelvic nerve and placed in a 10 ml bath superfused with Krebs solution (contents in millimoles: NaCl 120; KCl 5.9; NaH2PO4 1.2; MgSO4 1.2; NaHCO3 15.4; CaCl2 2.5; glucose 11.5), equilibrated with 95% O2 5% CO2. Both proximal and distal ends of the 30 mm length of bowel were secured to 8.5 F. three-way cannulae, and the lumen was perfused with Krebs solution. Ports on the cannulae were connected to a pressure transducer, large and small drainage tubing, and infusion tubing, which was connected, in turn, to a syringe driver (sp210iw; World Precision Instruments, Sarasota, Fla., USA). Following careful dissection of the pelvic nerve under the microscope into small branches, multi-fibre afferent activity was recorded using a glass suction electrode (tip diameter 50–100 µm) connected to a Neurolog headstage (NL 100; Digitimer Ltd., UK) and an AC amplifier (NL 104). Signals were amplified (×10,000), filtered (NL 125, band pass 200–4000 Hz) and captured by a computer via a power 1401 analogue-to-digital interface and Spike 2 software (version 4.03, Cambridge Electronic Design, UK). In all cases the tissues were allowed to stabilise in the bath for 60 min before the data were gathered. Branches that failed to respond consistently to repeated distensions were discarded. Control distensions to 40 mmHg with Krebs were repeated at 10 min intervals until nerve responses were stable. The various agonists were applied as a bolus to the serosal surface of the colorectum, and the antagonists were circulated via the pump into the organ bath.
The results for all experiments are presented as means ± SEMs. Data were compared by chi-squared test, Student’s t-test, or analysis of variance (ANOVA) as appropriate, and differences were considered statistically significant at P < 0.05.
Chemicals
ATP (disodium salt), α,β-meATP (lithium salt), 5-HT, pyridoxyl 5-phosphate 6-azophenyl-2′,4′-disulphonic acid (PPADS), suramin, tropisetron and uridine 5′-triphosphate (UTP) were all obtained from Sigma. All chemicals were diluted in Krebs solution to required concentrations before use. All drugs were prepared in deionised water as stock solutions, except for tropisetron, which was dissolved in dimethylsulphoxide (DMSO) to 1 mM, and were diluted to required concentrations before use. The maximum concentration of DMSO in the final solution (1%) did not affect responses.
Results
Increased responses to 5-HT in L6-S2 DRG neurons
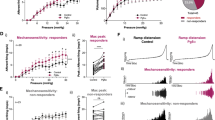
In wildtype mice, 100 µM ATP evoked a slowly desensitising inward current in 24% (13/33) of DRG neurons, with an average amplitude of 1.07 ± 0.12 nA (Figure 1). In 55% (18/33) of neurons, ATP evoked a rapidly desensitising current of 0.28 ± 0.02 nA (Figure 1A). A further 6% (2/33) of neurons gave biphasic responses with both transient and sustained components (Figure 1A). Comparable, transient, sustained or biphasic responses were evoked in these neurons by 100 µM α,β-meATP. Of wildtype DRG neurons, 63% (21/33) responded to 10 µM 5-HT with an inward current, with an amplitude of 0.87 ± 0.06 nA (Figure 1A). However, of the 5-HT-sensitive neurons, 62 ± 1.1% neurons responded to ATP (100 µM), while, in ATP-sensitive neurons, 88 ± 0.3% responded to 5-HT (10 µM), which indicated a high degree of overlap between ATP and 5-HT receptor expression. The amplitude of 5-HT evoked currents was 1.04 ± 0.07 nA and 0.27 ± 0.02 nA in ATP-sensitive (n = 9) and -insensitive (n = 7) neurons, respectively (Figure 1B). There was significant difference between them (P < 0.01, Student’s t-test). In contrast, the amplitude of ATP responses was similar on the 5-HT-sensitive and -insensitive neurons (Figure 1C). As shown in Figure 1C, I5-HT (10 µM) and IATP (100 µM) were significantly inhibited by tropisetron (0.3 µM) and suramin (100 µM), respectively. However, tropisetron had no effect on IATP as suramin did not alter I5-HT.
Response of isolated DRG neurons to ATP and 5-HT. A Traces showing the three types of currents evoked by ATP (100 µM; sustained, transient and biphasic) and current evoked by 5-HT (10 µM) on DRG neurons in wildtype (WT) mice. B Histograms showing the average of the amplitude of 5-HT response in ATP-sensitive [ATP(+)] and -insensitive [ATP (−))] neurons and average amplitude of ATP-evoked current in 5-HT-sensitive [5-HT(+)] and -insensitive [5-HT(−)] neurons. ** P<0.01, Student’s t-test. C Histograms showing the effect of tropisetron (0.3 µM) and suramin (100 µM) on the 5-HT and ATP-evoked responses on DRG neurons from WT mice. ** P<0.01, Student’s t-test.
In P2X3 −/− mice, application of α,β-meATP (100 µM) did not evoke any inward currents in DRG neurons (22 cells tested) (Figure 2A). No transient currents were evoked by ATP (100 µM) either. However, sustained responses to ATP were observed in 36% (8/22) of neurons, which was not significantly different from the proportion of neurons from wildtype mice (30%), which had a sustained component to the response (Figure 2D). In contrast, 89.3 ± 3% (n = 22) neurons responded to 5-HT (10 µM) with the amplitude of 0.94 ± 0.03 nA (Figure 2E). The percentage of neurons sensitive to 5-HT in P2X3 −/− mice was significantly higher than that in wildtype mice (P < 0.05) (Figure 2D), although there was no significant difference in the amplitude of 5-HT response in P2X3 wildtype and P2X3 −/− mice (P > 0.05) (Figure 2E).
Comparison of responses to ATP and 5-HT in knockout mice. A ATP evoked only sustained currents in DRG neurons from P2X3 −/− mice, while the transient response to 5-HT was quite normal. B ATP and α,β-meATP, like 5-HT, evoked only transient currents in DRG neurons from P2X2 −/− mice. C ATP and α,β-meATP failed to evoke any current in DRG neurons in P2X2/P2X3 Dbl−/− mice, while 5-HT evoked a significantly larger current. D Percentage of neurons responding to 5-HT (10 µM) from wildtype (WT) and the three knockout strains of mouse. E Average amplitude of currents evoked by 5-HT (10 µM) from WT and the three knockout strains of mouse did not differ between wildtype and knockout neurons. * P<0.05, Student’s t-test.
In P2X2 −/− mice, application of either ATP (100 µM) or α,β-meATP (100 µM) failed to produce a slowly desensitizing inward current in any of the DRG neurons (31 cells tested) (Figure 2B). Transient responses to ATP and α,β-meATP were observed in 58% (18/31) of neurons, which was a similar proportion to that in wildtype mice (Figure 2D). In 87.3 ± 3% (n = 23) neurons, 5-HT, 10 µM, evoked a response with a mean amplitude of 0.83 ± 0.06 nA (Figure 2E). The percentage of neurons responding to 5-HT was significantly higher than that in wildtype mice (P < 0.05) (Figure 2D), although the amplitude of 5-HT response was not significantly different in P2X2 wildtype and P2X2 −/− mice (P > 0.05) (Figure 2E).
In P2X2/P2X3 Dbl−/− mice there was no response to ATP (100 µM) or to α,β-meATP (100 µM) in 24 neurons tested (Figure 2C). In contrast, 90 ± 3% (n = 24; Figure 2D) neurons responded to 5-HT 10 µM with the amplitude of 1.68 ± 0.10 nA (Figure 2E). Both the percentage and amplitude of the 5-HT response in P2X2/P2X3 Dbl−/− mice were significantly higher than those in wildtype mice (P < 0.05, P < 0.05, Figure 2D,E), although the size of the neurons (as determined from measurement of membrane capacitance) were not significantly different.
The response to 5-HT was activated rapidly and declined in the continued presence of the agonist (Figure 2C).
Pelvic afferent activity from the colorectum in P2X2/P2X3 Dbl−/− and wildtype mice
To see if changes in receptor signalling at the cell body were mirrored by changes at the nerve terminal, we investigated afferent nerve activity in the colorectum from wildtype and knockout mice. Bolus application of α,β-meATP 100 µM in the wildtype preparation resulted in a rapid increase from baseline activity by 174 ± 10% (n = 4), but, in the knockout preparation, the acute response was completely absent. Bolus applications of ATP, 1 mM, to the serosal surface of the colorectum resulted in consistent rises in pelvic nerve activity in the wildtype preparations. The mean increase from baseline activity in the wildtype was 157 ± 33% (Figure 3A). In P2X2/P2X3 Dbl−/− preparations, a much slower and smaller increase in activity of 49 ± 11% (n = 4; P < 0.05) was observed. In four similar experiments application of the P2Y receptor agonist UTP to the colorectum also increased the nerve activity by 40 ± 3% in wildtype preparations and, to the greater extent of 76 ± 6%, in the P2X2/P2X3 Dbl−/− mice (P < 0.01; data not shown).
In the wildtype mice 10 µM 5-HT increased spike frequency in the pelvic nerve by 116 ± 15%. However, in the P2X2/P2X3 Dbl−/− preparations, the response was 183 ± 11% over baseline activity (n = 4), which was significantly greater than that in the wildtype preparation (P < 0.05) (Figure 3B).
Agonist-evoked responses in pelvic afferents in an in vitro colorectal preparation from wildtype (WT) and P2X2/P2X3 Dbl−/− mice. A Pooled data from four wildtype and four P2X2/P2X3 Dbl−/− preparations show that the afferent responses to 1 mM ATP was significantly attenuated in P2X2/P2X3 Dbl−/− preparations. * P<0.05, Student’s t-test. B Pooled data from four wildtype and four P2X2/P2X3 Dbl−/− preparations indicate an increased response to 5-HT in pelvic afferent fibres from P2X2/P2X3 Dbl−/− preparations. * P<0.05, Student’s t-test.
We also compared the responses to colorectal distension in P2X2/3 wildtype and knockout mice. Multi-fibre activity in the pelvic nerve was measured at eight different intraluminal pressures from 0 mmHg to 40 mmHg, and these values were plotted as a percentage of the (maximum) activity at 40 mmHg. Figure 4A shows that the relative activity at any given pressure was reduced in the P2X2/P2X3 Dbl−/− preparations. ANOVA demonstrated that these two plots were significantly different (P < 0.01). The effect of the P2 receptor antagonist PPADS, 100 µM, on the response to distension was tested in the two types of preparation. In the wildtype mice, distension-evoked activity was reduced by PPADS by 32 ± 2%, whereas, in the P2X2/P2X3 Dbl−/−, there was only a 9 ± 3% reduction (n = 4) (Figure 4B). This difference between wildtype and P2X2/P2X3 Dbl−/− preparations was highly statistically significant (P < 0.001). Because application of 5-HT had caused greater excitation of pelvic nerve fibres in the P2X2/P2X3 Dbl−/− compared to the wildtype, the effect of the 5-HT3 receptor antagonist tropisetron on the nerve response to distension was evaluated. Figure 4C shows examples of this. Application of tropisetron, 10 µM, resulted in a 40 ± 2% reduction in distension-evoked activity in the wildtype preparations, whereas, in the P2X2/P2X3 Dbl−/−, a more pronounced reduction of 53 ± 2% was observed (n = 4; Figure 4C). This difference was statistically significant (P < 0.01) (Figure 4D).
Distension-evoked responses of pelvic afferents from in vitro colorectal preparations from wildtype (WT) and P2X2/P2X3 Dbl−/− mice. (A) A plot of afferent activity (normalised as percentage of the peak activity at 40 mmHg) in relation to intraluminal pressure indicates reduced responsiveness of pelvic afferent to colorectal distension in the P2X2/P2X3 Dbl−/− preparations. (B) The P2 antagonist PPADS resulted in a greater reduction in the responses of pelvic afferents to colorectal distension in wildtype than in the P2X2/P2X3 Dbl−/− mice. *** P < 0.001, Student’s t-test. (C) Effects of tropisetron on the responses of pelvic afferents to colorectal distension in preparations from wildtype, where tropisetron produces a reversible decrease in the multi-unit discharge rate, and P2X2/P2X3 Dbl−/− mice, where tropisetron produces a more dramatic inhibition. (D) Pooled data from four wildtype and four P2X2/P2X3 Dbl−/− mice confirm a greater reduction in distension-induced afferent discharge in the P2X2/P2X3 Dbl−/− preparations. ** P < 0.01, Student’s t-test
Discussion
The pelvic nerves contain afferent fibres innervating pelvic organs such as the urinary bladder, the urethra and the colorectum. Pelvic afferents have their cell bodies in the DRG of the lower lumbar and sacral segments. Previous studies in this laboratory have suggested that extracellular ATP contributes significantly to mechanosensory transduction in the urinary bladder and in the colorectum [2–4]. In the present study we demonstrate that a high proportion of L6-S2 DRG neurons responded to 5-HT as well as ATP. Pelvic afferents in mice deficient in the P2X2 and P2X3 subunits had a reduced sensitivity to colorectal distension, whereas the afferent responses to 5-HT were increased in mice deficient in P2X2 and/or P2X3 receptor subunits.
In DRG neurons three types of responses to ATP have been observed: transient, sustained and biphasic (having both transient and sustained components), which suggest the presence of multiple P2X receptor subtypes (see [7]). Although in situ hybridisation studies have shown that the mRNAs for P2X1–6 are all expressed in sensory neurons [25], the current study is in keeping with the widely held view that P2X receptors containing the P2X2 and/or P2X3 subunits, namely homomeric P2X2, P2X3 and heteromeric P2X2/3 receptors, mediate these responses (see [7]). Thus, in P2X3 −/− mice, no transient response to ATP was detected, whereas, in P2X2 −/− mice, sustained responses to either ATP or α,β-meATP were absent. In P2X2/P2X3 Dbl−/− mice neither transient nor sustained responses to either ATP or α,β-meATP were detected. Because the rate of desensitisation of the P2X3 receptor is fast, compared with the speed of agonist application, there will be an underestimate of the amplitude of the P2X3 receptor-mediated response. However, this error will have been considerably reduced by the use of a high agonist concentration.
We have previously shown in a rat colorectal preparation that ATP and its stable analogue, α,β-meATP, could directly activate pelvic afferents and sensitise their responses to colorectal distension [2]. In contrast, the P2 receptor antagonist PPADS resulted in a reduction in the pelvic afferent responses to colorectal distension, suggesting that ATP contributes to mechanosensory transduction through interactions with P2X receptors. The present study provides further support for this. Firstly, we show that, in the P2X2/P2X3 Dbl−/− mice, the responses of pelvic afferent fibres to colorectal distension were reduced compared to those in the wildtype animals. Secondly, the P2 receptor antagonist PPADS resulted in a 32.1% reduction in distension-induced afferent activity in the wildtype but only 9.3% reduction in the knockout preparations, suggesting that P2X receptors containing the P2X2 and/or P2X3 subunits are involved in this purinergic mechanosensory transduction.
In the in vitro colorectal preparation from P2X2/P2X3 Dbl−/− mice, ATP still evoked an increase in pelvic afferent discharge. Although these responses might involve other P2X receptor subtypes, the slow kinetics of the response and the lack of effect of α,β-meATP would be in keeping with the involvement of metabotropic receptors, possibly adenosine receptors (i.e., P1) or P2Y receptors. ATP can easily be broken down to ADP, AMP and adenosine under the activity of ecto-nucleotidases, which are widely distributed in different tissues [26]. ADP is a potent agonist of certain P2Y receptors, whereas adenosine is an endogenous agonist of A1 receptors, which are present on afferent terminals. The observation that UTP evoked a greater increase in pelvic afferent discharge in P2X2/P2X3 Dbl−/− mice suggests that P2Y2, P2Y4 or P2Y11 receptor-mediated signalling might be up-regulated in these mice.
5-HT is recognised as an important mediator of afferent excitation in the gut [27, 28]. The primary source of 5-HT in the body is a population of enterochromaffin cells in the intestinal mucosa. In patients with irritable bowel syndrome (IBS) there was an increase in the population of enterochromaffin cells in the colon [29]. 5-HT activates vagal afferent endings in the upper gastrointestinal tract via 5-HT3 receptors [11, 12, 30]. More recently, Hicks et al. [10] demonstrated excitation of colonic afferent fibres in the hypogastric nerve by 5-HT, and the effects were partly mediated by 5-HT3 receptors. However, so far, there has been no direct demonstration of the effects of 5-HT on pelvic afferents of the colorectum. In this study we first showed that a high proportion of L6-S2 DRG neurons responded to 5-HT with a fast inward current and that bath application of 5-HT resulted in rapid excitation of the pelvic afferents of the colorectum. The responses to 5-HT in the DRG neurons were blocked by tropisetron, suggesting that 5-HT3 receptors mediated this response. This is consistent with previous in situ hybridisation and immunohistochemical data, which demonstrated the presence of 5-HT3 along with other 5-HT receptor subtypes in DRG neurons [17, 31]. Furthermore, we showed that tropisetron attenuated the responses of the pelvic afferents to colorectal distension. In vitro release of 5-HT from the colon has been shown before [32, 33]. The present results strongly suggest that 5-HT released during distension contributes to mechanosensory transduction in the colorectum.
5-HT can modulate primary afferent input to the spinal cord via the activation of multiple 5-HT receptor subtypes [34]. The rapid effects of 5-HT in mechanosensory transduction are mediated by 5HT3 receptors and are antagonised by tropisetron. Whether 5-HT has additional modulatory effects in mechanosensory transduction, mediated through other 5HT receptor subtypes, remains to be determined.
There appears to be a clear correlation between the P2X and 5-HT3-mediated signalling in pelvic afferent neurons. Firstly, there was a high degree of overlap in the neurons that respond to ATP or 5-HT, and the I5-HT was significantly greater in ATP-sensitive neurons than in ATP-insensitive neurons, indicating that more than half of the pelvic afferent neurons express both P2X and 5-HT3 receptors. Secondly, a greater proportion of L6-S2 DRG cells responded to 5-HT in mice deficient in P2X2 and/or P2X3 subunits, and the amplitude of 5-HT-induced currents was significantly higher in P2X2/P2X3 Dbl−/− than in the wildtype mice. The relationship between receptor activation and action potential discharge in a multi-unit recording is likely to be complex. Our results from dissociated DRG neurons suggest that there is not only an increase in the density of 5HT3 receptor expression but also an increase in the number of neurons expressing these receptors in P2X2/P2X3 Dbl−/− knockout mice. If these changes occur in vivo at the level of the afferent nerve terminals, then a greater number of afferent fibres will be activated by distension-evoked 5-HT release. Consequently, there will be a greater antagonism of the response by a high concentration of tropisetron. Thirdly, 5-HT evoked a greater increase in pelvic afferent discharge in the P2X2/P2X3 Dbl−/− colorectal preparation than in the wildtype preparations, and tropisetron resulted in a greater reduction in distension-induced afferent discharge in the knockouts than in the wildtype preparations. Previous evidence indicates that P2X receptors might interact with nicotinic acetylcholine and 5-HT3 receptors [35]. In the submucosal neurons of guinea pig ileum, simultaneous activation of P2X and 5-HT3 receptors resulted in mutual inhibition of ATP and 5-HT-induced currents [19, 20]. Thus, one possibility for the increased 5-HT signalling is by the removal of ATP-mediated inhibitory modulation of the 5-HT receptors. The other possibility is that, over the course of development of the P2X2/P2X3 Dbl−/− mice, there is an adaptive increase in the expression of 5-HT3 receptors.
In summary, our results suggest that P2X receptors and 5-HT3 receptors are functionally associated, such that the removal of P2X receptors results in adaptive increase in 5-HT3-mediated signalling in the pelvic afferent neurons.
Abbreviations
- α,β-meATP:
-
α,β-methylene ATP
- DRG:
-
dorsal root ganglion
- HBSS:
-
Hank’s balanced salt solution
- 5-HT:
-
5-hydroxytryptamine
- DMSO:
-
dimethylsulphoxide
- PPADS:
-
pyridoxyl 5-phosphate 6-azophenyl-2–4– disulphonic acid
References
Kirkup AJ, Booth CE, Chessell IP et al (1999) Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J Physiol 520:551–63
Wynn G, Rong W, Xiang Z, Burnstock G (2003) Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology 125:1398–409
Vlaskovska M, Kasakov L, Rong W et al (2001) P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21:5670–677
Rong W, Spyer KM, Burnstock G (2002) Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol 541:591–00
Burnstock G (1999) Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194:335–42
Burnstock G (2001) Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci 22:182–88
Dunn PM, Zhong Y, Burnstock G (2001) P2X receptors in peripheral neurones. Prog Neurobiol 65:107–34
Cockayne DA, Hamilton SG, Zhu Q-M et al (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407:1011–015
Bian X, Ren J, DeVries M, Schnegelsberg B et al (2003) Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J Physiol 551:309–22
Hicks GA, Coldwell JR, Schindler M et al (2002) Excitation of rat colonic afferent fibres by 5-HT3 receptors. J Physiol 544:861–69
Hillsley K, Grundy D (1998) Sensitivity to 5-hydroxytryptamine in different afferent subpopulations within mesenteric nerves supplying the rat jejunum. J Physiol 509:717–27
Hillsley K, Kirkup AJ, Grundy D (1998) Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol 506:551–61
Zeitz KP, Guy N, Malmberg AB et al (2002) The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 22:1010–019
Tecott LH, Maricq AV, Julius D (1993) Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci USA 90:1430–434
Vulchanova L, Riedl MS, Shuster SJ et al (1997) Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology 36:1229–242
Vulchanova L, Riedl MS, Shuster SJ et al (1998) P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci 10:3470–478
Morales M, McCollum N, Kirkness EF (2001) 5-HT3-receptor subunits A and B are coexpressed in neurons of the dorsal root ganglion. J Comp Neurol 438:163–72
Nicholson R, Small J, Dixon AK et al (2003) Serotonin receptor mRNA expression in rat dorsal root ganglion neurons. Neurosci Lett 337:119–22
Barajas-López C, Montaño LM, Espinosa-Luna R (2002) Inhibitory interactions between 5-HT3 and P2X channels in submucosal neurons. Am J Physiol Gastrointest Liver Physiol 283:G1238–G1248
Boué-Grabot É, Barajas-López C, Chakfe Y et al (2003) Intracellular cross talk and physical interaction between two classes of neurotransmitter-gated channels. J Neurosci 23:1246–253
Godecke A, Schrader J (2000) Adaptive mechanisms of the cardiovascular system in transgenic mice—lessons from eNOS and myoglobin knockout mice. Basic Res Cardiol 95:492–98
Cockayne D, Dunn PM, Zhong Y et al (2005) P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol 567:621–39
Rong W, Gourine AV, Cockayne DA et al (2003) Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci 23:11315–1321
Dunn PM, Liu M, Zhong Y et al (2000) Diinosine pentaphosphate: an antagonist which discriminates between recombinant P2X3 and P2X2/3 receptors and between two P2X receptors in rat sensory neurones. Br J Pharmacol 130:1378–384
Collo G, North RA, Kawashima E et al (1996) Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci 16:2495–507
Zimmermann H (2001) Ecto-nucleotidases. In: Abbracchio MP, Williams M (eds) Handbook of experimental pharmacology. Purinergic and pyrimidergic signalling. Springer, Heidelberg, pp 209–50
Camilleri M (2002) Serotonergic modulation of visceral sensation: lower gut. Gut 51:181–86
Tack J, Sarnelli G (2002) Serotonergic modulation of visceral sensation: upper gastrointestinal tract. Gut 51:177–80
Spiller RC, Jenkins D, Thornley JP et al (2000) Increased rectal mucosal enteroendocrine cells, T lymphocytes and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47:804–11
Blackshaw LA, Grundy D (1993) Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. J Auton Nerv Sys 45:41–0
Chen JJ, Vasko MR, Wu X et al (1998) Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. J Pharmacol Exp Ther 287:1119–127
Kirchgessner AL, Tamir H, Gershon MD (1992) Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci 12:235–48
Pan H, Gershon MD (2000) Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci 20:3295–309
Garraway SM, Hochman S (2001) Pharmacological characterization of serotonin receptor subtypes modulating primary afferent input to deep dorsal horn neurons in the neonatal rat. Br J Pharmacol 132:1789–798
Zhou X, Galligan JJ (1998) Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J Physiol 513:685–97
Acknowledgement
The authors thank Dr. Gillian E. Knight for editorial assistance. We much appreciate the generous gift from Debra Cockayne and Anthony Ford at Roche Palo Alto of the P2X2 and P2X3 knockout mice used in this study. This work was supported by The Wellcome Trust and Roche Palo Alto.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ma, B., Wynn, G., Dunn, P.M. et al. Increased 5-HT3-mediated signalling in pelvic afferent neurons from mice deficient in P2X2 and/or P2X3 receptor subunits. Purinergic Signalling 2, 481–489 (2006). https://doi.org/10.1007/s11302-006-9017-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-006-9017-z