Abstract
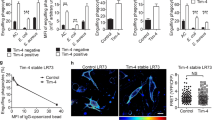
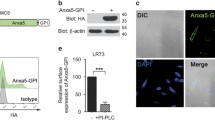
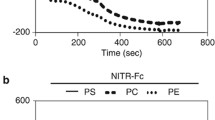
The P2X7 receptor is widely recognized to mediate the proinflammatory effects of extracellular ATP. However this receptor in the absence of ATP may have a function unrelated to inflammation. Our data show that P2X7 expressed on the surface of monocyte/macrophages or on epithelial HEK-293 cells greatly augments the engulfment of latex beads and live and heat-killed bacteria by effector phagocyte in the absence of ATP and serum. The expression of P2X7 on the effector also confers the ability to phagocytose apoptotic target cells and an accumulation of P2X7 can be seen at the attachment point to the target. Activation of the P2X7 receptor by ATP causes a slow dissociation (over 10–15 min) of nonmuscle myosin from the P2X7 membrane complex and abolishes further P2X7-mediated phagocytosis of these targets. The recent crystal structure of the homologous zebrafish P2X4 receptor shows an exposed “nose” of the ectodomain (residues 115–162) which contains three of the five disulfide bonds conserved in all P2X receptors. Three short biotin-labeled peptides mimicking sequence of this exposed region bound to apoptotic target cells but not to either viable cells or to other target particles. All three peptides contained one or two cysteine residues and their replacement by alanine abolished peptide binding. These data implicate thiol-disulfide exchange reactions in the initial tethering of apoptotic cells to macrophage and establish P2X7 as one of the scavenger receptors involved in the recognition and removal of apoptotic cells in the absence of extracellular ATP and serum.







Similar content being viewed by others
References
Chen G, Zhuchenko O, Kuspa A (2007) Immune-like phagocyte activity in the social amoeba. Science 317(5838):678–681
Herbomel P, Thisse B, Thisse C (1999) Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 126(17):3735–3745
Brodsky IE, Medzhitov R (2009) Targeting of immune signalling networks by bacterial pathogens. Nat Cell Biol 11(5):521–526
Ravichandran KS, Lorenz U (2007) Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol 7(12):964–974
Cvetanovic M, Mitchell JE, Patel V et al (2006) Specific recognition of apoptotic cells reveals a ubiquitous and unconventional innate immunity. J Biol Chem 281(29):20055–20067
Vergeer M, Korporaal SJ, Franssen R et al (2011) Genetic variant of the scavenger receptor BI in humans. N Engl J Med 364(2):136–145
Kristiansen M, Graversen JH, Jacobsen C et al (2001) Identification of the haemoglobin scavenger receptor. Nature 409(6817):198–201
Baranova IN, Bocharov AV, Vishnyakova TG et al (2010) CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAA-induced signaling in human and rodent cells. J Biol Chem 285(11):8492–8506
Stuart LM, Deng J, Silver JM et al (2005) Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J Cell Biol 170(3):477–485
Fabriek BO, van Bruggen R, Deng DM et al (2009) The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 113(4):887–892
Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC (2002) Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia 40(2):195–205
Pluddemann A, Neyen C, Gordon S (2007) Macrophage scavenger receptors and host-derived ligands. Methods 43(3):207–217
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S (2007) Identification of Tim4 as a phosphatidylserine receptor. Nature 450(7168):435–439
Santiago C, Ballesteros A, Martinez-Munoz L et al (2007) Structures of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent ligand binding site where phosphatidylserine binds. Immunity 27(6):941–951
Kobayashi N, Karisola P, Pena-Cruz V et al (2007) TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27(6):927–940
Park D, Tosello-Trampont AC, Elliott MR et al (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450(7168):430–434
Park SY, Jung MY, Kim HJ et al (2008) Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ 15(1):192–201
Scott RS, McMahon EJ, Pop SM et al (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411(6834):207–211
Ishimoto Y, Ohashi K, Mizuno K, Nakano T (2000) Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem 127(3):411–417
Hanayama R, Tanaka M, Miyasaka K et al (2004) Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304(5674):1147–1150
Gardai SJ, McPhillips KA, Frasch SC et al (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123(2):321–334
Touret N, Paroutis P, Terebiznik M et al (2005) Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell 123(1):157–170
Steinkamp JA, Wilson JS, Saunders GC, Stewart CC (1982) Phagocytosis: flow cytometric quantitation with fluorescent microspheres. Science 215(4528):64–66
Gu BJ, Saunders BM, Jursik C, Wiley JS (2010) The P2X7-nonmuscle myosin membrane complex regulates phagocytosis of non-opsonized particles and bacteria by a pathway attenuated by extracellular ATP. Blood 115(8):1621–1631
Cvetanovic M, Ucker DS (2004) Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol 172(2):880–889
Solle M, Labasi J, Perregaux DG et al (2001) Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 276(1):125–132
Komohara Y, Terasaki Y, Kaikita K, Suzuki H, Kodama T, Takeya M (2005) Clearance of apoptotic cells is not impaired in mouse embryos deficient in class A scavenger receptor types I and II (CD204). Dev Dyn 232(1):67–74
Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL (2006) Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med 203(12):2613–2625
Gu BJ, Saunders BM, Petrou S, Wiley JS (2011) P2X7 is a scavenger receptor for apoptotic cells in the absence of its ligand extracellular ATP. J Immunol 187(5):2365–2375
Kuehnel MP, Rybin V, Anand PK, Anes E, Griffiths G (2009) Lipids regulate P2X7-receptor-dependent actin assembly by phagosomes via ADP translocation and ATP synthesis in the phagosome lumen. J Cell Sci 122(Pt 4):499–504
Marina-Garcia N, Franchi L, Kim YG et al (2008) Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol 180(6):4050–4057
McDermott MF, Tschopp J (2007) From inflammasomes to fevers, crystals and hypertension: how basic research explains inflammatory diseases. Trends in molecular medicine 13(9):381–388
Kusner DJ, Barton JA (2001) ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J Immunol 167(6):3308–3315
Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA (2001) ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X(7)-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol 167(6):3300–3307
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82(4):1013–1067
Kawate T, Michel JC, Birdsong WT, Gouaux E (2009) Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460(7255):592–598
Young MT (2010) P2X receptors: dawn of the post-structure era. Trends Biochem Sci 35(2):83–90
Obeid M, Tesniere A, Panaretakis T et al (2007) Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev 220:22–34
Freeman M, Ashkenas J, Rees DJ et al (1990) An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci U S A 87(22):8810–8814
Shu S, Liu X, Korn ED (2005) Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium. Proc Natl Acad Sci USA 102(5):1472–1477
Tsai RK, Discher DE (2008) Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol 180(5):989–1003
Gu BJ, Rathsam C, Stokes L, McGeachie AB, Wiley JS (2009) Extracellular ATP dissociates nonmuscle myosin from P2X7 complex: this dissociation regulates P2X7 pore formation. Am J Physiol Cell Physiol 297(2):C430–C439
Acknowledgments
We thank the National Health & Medical Research Council of Australia and the Leukaemia Foundation of Australia for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiley, J.S., Gu, B.J. A new role for the P2X7 receptor: a scavenger receptor for bacteria and apoptotic cells in the absence of serum and extracellular ATP. Purinergic Signalling 8, 579–586 (2012). https://doi.org/10.1007/s11302-012-9308-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-012-9308-5