Abstract
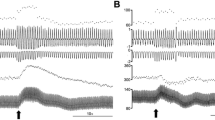
P2X receptors are expressed on ventrolateral medulla projecting paraventricular nucleus (PVN) neurons. Here, we investigate the role of adenosine 5′-triphosphate (ATP) in modulating sympathetic nerve activity (SNA) at the level of the PVN. We used an in situ arterially perfused rat preparation to determine the effect of P2 receptor activation and the putative interaction between purinergic and glutamatergic neurotransmitter systems within the PVN on lumbar SNA (LSNA). Unilateral microinjection of ATP into the PVN induced a dose-related increase in the LSNA (1 nmol: 38 ± 6 %, 2.5 nmol: 72 ± 7 %, 5 nmol: 96 ± 13 %). This increase was significantly attenuated by blockade of P2 receptors (pyridoxalphosphate-6-azophenyl-20,40-disulphonic acid, PPADS) and glutamate receptors (kynurenic acid, KYN) or a combination of both. The increase in LSNA elicited by L-glutamate microinjection into the PVN was not affected by a previous injection of PPADS. Selective blockade of non-N-methyl-D-aspartate receptors (6-cyano-7-nitroquinoxaline-2,3-dione disodium salt, CNQX), but not N-methyl-D-aspartate receptors (NMDA) receptors (DL-2-amino-5-phosphonopentanoic acid, AP5), attenuated the ATP-induced sympathoexcitatory effects at the PVN level. Taken together, our data show that purinergic neurotransmission within the PVN is involved in the control of SNA via P2 receptor activation. Moreover, we show an interaction between P2 receptors and non-NMDA glutamate receptors in the PVN suggesting that these functional interactions might be important in the regulation of sympathetic outflow.






Similar content being viewed by others
Abbreviations
- ATP:
-
Adenosine 5′-triphosphate
- PPADS:
-
Pyridoxalphosphate-6-azophenyl-20,40-disulphonic acid
- α,β-meATP:
-
α,β-MethyleneATP
- L-glu:
-
L-Glutamate
- KYN:
-
Kynurenic acid
- AP5:
-
DL-2-Amino-5-phosphonopentanoic acid
- CNQX:
-
6-Cyano-7-nitroquinoxaline-2,3-dione disodium salt
References
Swanson LW, Sawchenko PE (1983) Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6:269–324
Dampney RA (1994) Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74:323–364
Coote JH, Yang Z, Pyner S, Deering J (1998) Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol 25:461–463
Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM (2005) Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol 32:419–425
Guyenet PG (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7:335–346
Dampney RA, Czachurski J, Dembowsky K, Goodchild AK, Seller H (1987) Afferent connections and spinal projections of the pressor region in the rostral ventrolateral medulla of the cat. J Auton Nerv Syst 20:73–86
Yang Z, Coote JH (1998) Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol 513:521–530
Hardy SG (2001) Hypothalamic projections to cardiovascular centers of the medulla. Brain Res 894:233–240
Swanson LW, Kuypers HG (1980) The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194:555–570
Sawchenko PE, Swanson LW (1982) Immunohistochemical 546 identification of neurones in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205:260–272
Lovick TA, Coote JH (1988) Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res 454:123–130
Shafton AD, Ryan A, Badoer E (1998) Neurones in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801:239–243
Pyner S, Coote JH (1999) Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurones. Neuroscience 88:949–957
Badoer E (2001) Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 28:95–99
Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV (1994) Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol 266:R306–313
Stern JE, Li Y, Zhang W (2003) Nitric oxide: a local signalling molecule controlling the activity of pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Acta Physiol Scand 177:37–42
Li Y, Zhang W, Stern JE (2003) Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurones that innervate the medulla oblongata: role of GABA. Neuroscience 118:585–601
Allen AM (2002) Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39:275–280
Silva AQ, Santos RA, Fontes MA (2005) Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension 46:341–348
Kannan H, Hayashida Y, Yamashita H (1989) Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol 256:R1325–1330
Busnardo C, Tavares RF, Corrêa FM (2009) Role of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors in the cardiovascular effects of L-glutamate microinjection into the hypothalamic paraventricular nucleus of unanesthetized rats. J Neurosci Res 87:2066–2077
Busnardo C, Crestani CC, Tavares RF, Resstel LB, Correa FM (2010) Cardiovascular responses to L-glutamate microinjection into the hypothalamic paraventricular nucleus are mediated by a local nitric oxide-guanylate cyclase mechanism. Brain Res 1344:87–95
Chen QH, Haywood JR, Toney GM (2003) Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension 42:725–731
Chen QH, Toney GM (2003) Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285:R1231–1239
Zhang K, Patel KP (1998) Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol 275:R728–34
Kapoor JR, Sladek CD (2000) Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J Neurosci 20:8868–8875
Abbracchio MP, Burnstock G (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 64:445–475
Baldwin SA, Mackey JR, Cass CE, Young JD (1999) Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today 5:216–224
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H (1999) Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol 514:351–367
Yao ST, Gourine AV, Spyer KM, Barden JA, Lawrence AJ (2003) Localisation of P2X2 receptor subunit immunoreactivity on nitric oxide synthase expressing neurones in the brain stem and hypothalamus of the rat: a fluorescence immunohistochemical study. Neuroscience 121:411–419
Cham JL, Owens NC, Barden JA, Lawrence AJ, Badoer E (2006) P2X purinoceptor subtypes on paraventricular nucleus neurones projecting to the rostral ventrolateral medulla in the rat. Exp Physiol 91:403–411
Guo W, Sun J, Xu X, Bunstock G, He C, Xiang Z (2009) P2X receptors are differentially expressed on vasopressin- and oxytocin-containing neurones in the supraoptic and paraventricular nuclei of rat hypothalamus. Histochem Cell Biol 131:29–41
de Paula PM, Antunes VR, Bonagamba LG, Machado BH (2004) Cardiovascular responses to microinjection of ATP into the nucleus tractus solitarii of awake rats. Am J Physiol Regul Integr Comp Physiol 287:R1164–1171
Antunes VR, Bonagamba LG, Machado BH (2005) Hemodynamic and respiratory responses to microinjection of ATP into the intermediate and caudal NTS of awake rats. Brain Res 1032:85–93
Antunes VR, Braga VA, Machado BH (2005) Autonomic and respiratory responses to microinjection of ATP into the intermediate or caudal nucleus tractus solitarius in the working heart–brainstem preparation of the rat. Clin Exp Pharmacol Physiol 32:467–472
Yao ST, Lawrence AJ (2005) Purinergic modulation of cardiovascular function in the rat locus coeruleus. Br J Pharmacol 145:342–352
Braga VA, Soriano RN, Braccialli AL, de Paula PM, Bonagamba LG, Paton JF et al (2007) Involvement of L-glutamate and ATP in the neurotransmission of the sympathoexcitatory component of the chemoreflex in the commissural nucleus tractus solitarii of awake rats and in the working heart-brainstem preparation. J Physiol 581:1129–1145
Cruz JC, Bonagamba LG, Machado BH (2010) Modulation of arterial pressure by P2 purinoceptors in the paraventricular nucleus of the hypothalamus of awake rats. Auton Neurosci 158:79–85
Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O (1998) A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurones of the rat hippocampus. Eur J Neurosci 10:3898–3902
Antunes VR, Yao ST, Pickering AE, Murphy D, Paton JF (2006) A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J Physiol 576:569–583
Paxinos G, Watson C (1996) The rat brain in stereotaxic coordinates. Academic, New York
Lash JM, Haase E, Shoukass AA (1992) Systemic responses to carotid 645 occlusion in the anesthetized rat. J Appl Physiol 72:1247–1254
Colombari DS, Colombari E, Freiria-Oliveira AH, Antunes VR, Yao ST, Hindmarch C, Ferguson AV, Fry M, Murphy D, Paton JFR (2011) Switching control of sympathetic activity from forebrain to hindbrain in chronic dehydration. J Physiol 589:4457–4471
Mori M, Tsushima H, Matsuda T (1992) Antidiuretic effects of purinoceptor agonists injected into the hypothalamic paraventricular nucleus of water-loaded, ethanol-anesthetized rats. Neuropharmacology 31:585–592
Song Z, Sladek CD (2006) Site of ATP and phenylephrine synergistic stimulation of vasopressin release from the hypothalamo-neurohypophyseal system. J Neuroendocrinol 18:266–272
Knott TK, Marrero HG, Custer EE, Lemos JR (2008) Endogenous ATP potentiates only vasopressin secretion from neurohypophysial terminals. J Cell Physiol 217:155–161
Li DP, Chen SR, Pan HL (2010) Adenosine inhibits paraventricular pre-sympathetic neurones through ATP-dependent potassium channels. J Neurochem 113:530–542
Chen ZP, Levy A, Lightman SL (1994) Activation of specific ATP receptors induces a rapid increase in intracellular calcium ions in rat hypothalamic neurones. Brain Res 641:249–256
Kubo T, Yanagihara Y, Yamaguchi H, Fukumori R (1997) Excitatory amino acid receptors in the paraventricular hypothalamic nucleus mediate pressor response induced by carotid body chemoreceptor stimulation in rats. Clin Exp Hypertens 19:1117–1134
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Ralevic V, Thomas T, Burnstock G, Spyer KM (1999) Characterization of P2 receptors modulating neural activity in rat rostral ventrolateral medulla. Neuroscience 94:867–878
Horiuchi J, Potts PD, Tagawa T, Dampney RA (1999) Effects of activation and blockade of P2x receptors in the ventrolateral medulla on arterial pressure and sympathetic activity. J Auton Nerv Syst 76:118–126
Pankratov Y, Lalo UV, Krishtal OA, Verkhratsky A (2002) Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J Physiol 542:529–536
Pankratov Y, Lalo U, Verkhratsky A, North RA (2007) Quantal release of ATP in mouse cortex. J Gen Physiol 129:257–265
Passamani LM, Pedrosa DF, Mauad H, Schenberg LC, Paton JF, Sampaio KN (2011) Involvement of the purinergic system in central cardiovascular modulation at the level of the nucleus ambiguus of anaesthetized rats. Exp Physiol 96:262–274
Scislo TJ, O’Leary DS (2000) Differential role of ionotropic glutamatergic mechanisms in responses to NTS P(2x) and A(2a) receptor stimulation. Am J Physiol Heart Circ Physiol 278:H2057–2068
Khakh BS, Gittermann D, Cockayne DA, Jones A (2003) ATP modulation of excitatory synapses onto interneurons. J Neurosci 23:7426–7437
Nieber K, Poelchen W, Illes P (1997) Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. Br J Pharmacol 122:423–430
Acknowledgments
This study was supported by Sao Paulo Research Foundation (FAPESP): #07/04085-0 and 10/17997-0. Ferreira-Neto HC is a recipient of a FAPESP fellowship #10/05037-1.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferreira-Neto, H.C., Yao, S.T. & Antunes, V.R. Purinergic and glutamatergic interactions in the hypothalamic paraventricular nucleus modulate sympathetic outflow. Purinergic Signalling 9, 337–349 (2013). https://doi.org/10.1007/s11302-013-9352-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-013-9352-9