Abstract
Introduction
It is challenging to establish the mechanisms involved in the variety of well-defined clinical phenotypes in autism spectrum disorder (ASD) and the pathways involved in their pathogeneses.
Objectives
The aim of the present study was to evaluate the metabolomic profiles of children with ASD subclassified by mental regression (AR) phenotype and with no regression (ANR).
Methods
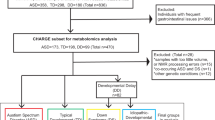
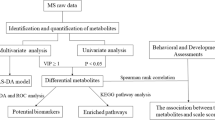
The present study was a cross-sectional case–control study. Thirty children aged 2–6 years with ASD were included: 15 with ANR and 15 with AR. In addition, a control group of 30 normally developing children was selected and matched to the ASD group by sex and age. Plasma samples were analyzed with a metabolomics single platform methodology based on liquid chromatography-mass spectrometry. Univariate and multivariate analysis, including orthogonal partial least squares-discriminant analysis modeling and Shared-and-Unique-Structures plots, were performed using MetaboAnalyst 4.0 and SIMCA-P 15. The primary endpoint was the metabolic signature profiling among healthy children and autistic children and their subgroups.
Results
Metabolomic profiles of 30 healthy children, 15 ANR and 15 AR were compared. Several differences between healthy children and children with ASD were detected, involving mainly amino acid, lipid and nicotinamide metabolism. Furthermore, we report subtle differences between the ANR and AR groups.
Conclusions
In this study, we report, for the first time, the plasmatic metabolomic profiles of children with ASD, including two different phenotypes based on mental regression status. The use of a liquid chromatography-mass spectrometry platform approach for metabolomics in ASD children using plasma appears to be very efficient and adds further support to previous findings in urine. Furthermore, the present study documents several changes related to amino acid, NAD+ and lipid metabolism that, in some cases, such as arginine and glutamate pathway alterations, seem to be associated with the AR phenotype. Further targeted analyses are needed in a larger cohort to validate the results presented herein.


Similar content being viewed by others
Data availability
The metabolomics datasets used and/or analyzed during the current study are included as Supplemental Material and any additional data is available from the corresponding author on reasonable request.
References
Adams, J. B., Audhya, T., McDonough-Means, S., Rubin, R. A., Quig, D., Geis, E., et al. (2011). Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutrition & Metabolism, 8(1), 34. https://doi.org/10.1186/1743-7075-8-34.
Alabdali, A., Al-Ayadhi, L., & El-Ansary, A. (2014). Association of social and cognitive impairment and biomarkers in autism spectrum disorders. Journal of Neuroinflammation, 11(1), 4. https://doi.org/10.1186/1742-2094-11-4.
Altieri, L., Neri, C., Sacco, R., Curatolo, P., Benvenuto, A., Muratori, F., et al. (2011). Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers. https://doi.org/10.3109/1354750x.2010.548010.
American Psychiatric Publishing, I. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). Arlington: American Psychiatric Publishing Inc.
Barnea-Goraly, N., Kwon, H., Menon, V., Eliez, S., Lotspeich, L., & Reiss, A. L. (2004). White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biological Psychiatry, 55(3), 323–326. https://doi.org/10.1016/j.biopsych.2003.10.022.
Bitar, T., Mavel, S., Emond, P., Nadal-Desbarats, L., Lefèvre, A., Mattar, H., et al. (2018). Identification of metabolic pathway disturbances using multimodal metabolomics in autistic disorders in a Middle Eastern population. Journal of Pharmaceutical and Biomedical Analysis, 152, 57–65. https://doi.org/10.1016/j.jpba.2018.01.007.
Delwing, D., Delwing, D., Bavaresco, C. S., & Wyse, A. T. S. (2008). Protective effect of nitric oxide synthase inhibition or antioxidants on brain oxidative damage caused by intracerebroventricular arginine administration. Brain Research, 1193, 120–127. https://doi.org/10.1016/j.brainres.2007.11.052.
Diémé, B., Mavel, S., Blasco, H., Tripi, G., Bonnet-Brilhault, F., Malvy, J., et al. (2015). Metabolomics study of urine in autism spectrum disorders using a multiplatform analytical methodology. Journal of Proteome Research, 14(12), 5273–5282. https://doi.org/10.1021/acs.jproteome.5b00699.
El-Ansary, A. K., Ben Bacha, A. G., & Al- Ayahdi, L. Y. (2011). Plasma fatty acids as diagnostic markers in autistic patients from Saudi Arabia. Lipids in Health and Disease, 10(1), 62. https://doi.org/10.1186/1476-511X-10-62.
Evans, A. M., Bridgewater, B. R., Liu, Q., Mitchell, M. W., Robinson, R. J., Dai, H., et al. (2014). High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Journal of Postgenomics Drug & Biomarker Development. https://doi.org/10.4172/2153-0769.1000132.
Evans, A. M., DeHaven, C. D., Barrett, T., Mitchell, M., & Milgram, E. (2009). Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical Chemistry, 81(16), 6656–6667. https://doi.org/10.1021/ac901536h.
Frye, R. E., Melnyk, S., & MacFabe, D. F. (2013). Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Translational Psychiatry, 3(1), e220. https://doi.org/10.1038/tp.2012.143.
Fukushima, T., Tawara, T., Isobe, A., Hojo, N., Shiwaku, K., & Yamane, Y. (1995). Radical formation site of cerebral complex I and Parkinson’s disease. Journal of Neuroscience Research, 42, 385–390.
Gabriele, S., Sacco, R., Cerullo, S., Neri, C., Urbani, A., Tripi, G., et al. (2014). Urinary p-cresol is elevated in young French children with autism spectrum disorder: A replication study. Biomarkers. https://doi.org/10.3109/1354750x.2014.936911.
Garcia-Aloy, M., Llorach, R., Urpi-Sarda, M., Jáuregui, O., Corella, D., Ruiz-Canela, M., et al. (2015). A metabolomics-driven approach to predict cocoa product consumption by designing a multimetabolite biomarker model in free-living subjects from the PREDIMED study. Molecular Nutrition & Food Research, 59(2), 212–220. https://doi.org/10.1002/mnfr.201400434.
Gevi, F., Zolla, L., Gabriele, S., & Persico, A. M. (2016). Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Molecular Autism, 7(1), 47. https://doi.org/10.1186/s13229-016-0109-5.
Goldani, A. A. S., Downs, S. R., Widjaja, F., Lawton, B., & Hendren, R. L. (2014). Biomarkers in autism. Frontiers in Psychiatry, 5, 100. https://doi.org/10.3389/fpsyt.2014.00100.
Howes, O. D., Rogdaki, M., Findon, J. L., Wichers, R. H., Charman, T., King, B. H., et al. (2018). Autism spectrum disorder: Consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. Journal of Psychopharmacology. https://doi.org/10.1177/0269881117741766.
Kałużna-Czaplińska, J., Jóźwik-Pruska, J., Chirumbolo, S., & Bjørklund, G. (2017). Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metabolic Brain Disease, 32(5), 1585–1593. https://doi.org/10.1007/s11011-017-0045-x.
Khaselev, N., & Murphy, R. C. (2000). Structural characterization of oxidized phospholipid products derived from arachidonate-containing plasmenyl glycerophosphocholine. Journal of Lipid Research, 41(4), 564–572.
Kim, S. H., & Lord, C. (2012). New autism diagnostic interview-revised algorithms for toddlers and young preschoolers from 12 to 47 months of age. Journal of Autism and Developmental Disorders, 42(1), 82–93. https://doi.org/10.1007/s10803-011-1213-1.
Lussu, M., Noto, A., Masili, A., Rinaldi, A. C., Dessì, A., De Angelis, M., et al. (2017). The urinary 1H-NMR metabolomics profile of an italian autistic children population and their unaffected siblings. Autism Research, 10(6), 1058–1066. https://doi.org/10.1002/aur.1748.
Mak-Fan, K. M., Morris, D., Vidal, J., Anagnostou, E., Roberts, W., & Taylor, M. J. (2013). White matter and development in children with an autism spectrum disorder. Autism, 17(5), 541–557. https://doi.org/10.1177/1362361312442596.
Mavel, S., Nadal-Desbarats, L., Blasco, H., Bonnet-Brilhault, F., Barthélémy, C., Montigny, F., et al. (2013). 1H-13C NMR-based urine metabolic profiling in autism spectrum disorders. Talanta, 114, 95–102. https://doi.org/10.1016/j.talanta.2013.03.064.
Ming, X., Stein, T. P., Barnes, V., Rhodes, N., & Guo, L. (2012). Metabolic perturbance in autism spectrum disorders: A metabolomics study. Journal of Proteome Research, 11(12), 5856–5862. https://doi.org/10.1021/pr300910n.
Mussap, M., Noto, A., & Fanos, V. (2016). Metabolomics of autism spectrum disorders: Early insights regarding mammalian-microbial cometabolites. Expert Review of Molecular Diagnostics, 16(8), 869–881. https://doi.org/10.1080/14737159.2016.1202765.
Nadal-Desbarats, L., Aïdoud, N., Emond, P., Blasco, H., Filipiak, I., Sarda, P., et al. (2014). Combined 1H-NMR and 1H-13C HSQC-NMR to improve urinary screening in autism spectrum disorders. Analyst, 139(13), 3460–3468. https://doi.org/10.1039/c4an00552j.
Napolioni, V., Persico, A. M., Porcelli, V., & Palmieri, L. (2011). The mitochondrial aspartate/glutamate carrier AGC1 and calcium homeostasis: Physiological links and abnormalities in autism. Molecular Neurobiology, 44(1), 83–92. https://doi.org/10.1007/s12035-011-8192-2.
Noto, A., Fanos, V., Barberini, L., Grapov, D., Fattuoni, C., Zaffanello, M., et al. (2014). The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. The Journal of Maternal-Fetal & Neonatal Medicine, 27(sup2), 46–52. https://doi.org/10.3109/14767058.2014.954784.
Palmieri, L., Papaleo, V., Porcelli, V., Scarcia, P., Gaita, L., Sacco, R., et al. (2010). Altered calcium homeostasis in autism-spectrum disorders: Evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Molecular Psychiatry, 15(1), 38–52. https://doi.org/10.1038/mp.2008.63.
Pastural, É., Ritchie, S., Lu, Y., Jin, W., Kavianpour, A., Khine Su-Myat, K., et al. (2009). Novel plasma phospholipid biomarkers of autism: Mitochondrial dysfunction as a putative causative mechanism. Prostaglandins Leukotrienes and Essential Fatty Acids, 81(4), 253–264. https://doi.org/10.1016/j.plefa.2009.06.003.
Rolf, L. H., Haarmann, F. Y., Grotemeyer, K. H., & Kehrer, H. (1993). Serotonin and amino acid content in platelets of autistic children. Acta Psychiatrica Scandinavica, 87(5), 312–316.
Rossignol, D. A., & Frye, R. E. (2012). Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Molecular Psychiatry, 17(3), 290–314. https://doi.org/10.1038/mp.2010.136.
Schmedes, M., Aadland, E. K., Sundekilde, U. K., Jacques, H., Lavigne, C., Graff, I. E., et al. (2016). Lean-seafood intake decreases urinary markers of mitochondrial lipid and energy metabolism in healthy subjects: Metabolomics results from a randomized crossover intervention study. Molecular Nutrition & Food Research, 60(7), 1661–1672. https://doi.org/10.1002/mnfr.201500785.
Wang, H., Liang, S., Wang, M., Gao, J., Sun, C., Wang, J., et al. (2016). Potential serum biomarkers from a metabolomics study of autism. Journal of Psychiatry and Neuroscience, 41(1), 27–37. https://doi.org/10.1503/jpn.140009.
West, P. R., Amaral, D. G., Bais, P., Smith, A. M., Egnash, L. A., Ross, M. E., et al. (2014). Metabolomics as a tool for discovery of biomarkers of autism spectrum disorder in the blood plasma of children. PLoS ONE, 9(11), e112445. https://doi.org/10.1371/journal.pone.0112445.
Willets, J. M., Lunec, J., Williams, A. C., & Griffiths, H. R. (1993). Neurotoxicity of nicotinamide derivatives: Their role in the aetiology of Parkinson’s disease. Biochemical Society Transactions, 21(Pt 3), 299S.
World Health Organization. (1992). The ICD-10 classification of mental and behavioural disorders. International Classification, 10, 1–267. https://doi.org/10.1002/1520-6505(2000)9:5%3c201:AID-EVAN2%3e3.3.CO;2-P.
Xia, J., Sinelnikov, I. V., Han, B., & Wishart, D. S. (2015). MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Research. https://doi.org/10.1093/nar/gkv380.
Yap, I. K. S., Angley, M., Veselkov, K. A., Holmes, E., Lindon, J. C., & Nicholson, J. K. (2010). Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. Journal of Proteome Research, 9(6), 2996–3004. https://doi.org/10.1021/pr901188e.
Acknowledgements
We gratefully acknowledge the patients, controls and family members who participated in this study.
Funding
ODRH has received funding from the European Union Seventh Framework Programme (FP7-PEOPLE-2013-COFUND) under grant agreement n° 609020 - Scientia Fellows.
Author information
Authors and Affiliations
Contributions
ODRH conducted the metabolomics data analyses, analyzed and interpreted the biochemical data and wrote the manuscript. AGF, MJTA, collected the plasma samples, interpreted the data. MGC and AG designed the study. JLPN, KFR, PMB were responsible of the clinical assessments and interpreted the data. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The present study was a cross-sectional case–control study case–control study and was approved by the Clinical Research and Bioethics Committee at Reina Sofia University Hospital respecting the fundamental principles established in the Declaration of Helsinki of 1964.
Informed consent
Informed consent from the children’s legal guardians was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rangel-Huerta, O.D., Gomez-Fernández, A., de la Torre-Aguilar, M.J. et al. Metabolic profiling in children with autism spectrum disorder with and without mental regression: preliminary results from a cross-sectional case–control study. Metabolomics 15, 99 (2019). https://doi.org/10.1007/s11306-019-1562-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-019-1562-x