Abstract
Purpose
The aim of the study was to improve abdominal tumor detection by use of a dual radiologic contrast protocol.
Procedures
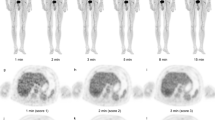
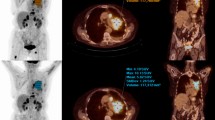
eXia160® (Benitio international) was mixed with 2-deoxy-2-[18F]fluoro-d-glucose or 3′-[18F]fluoro-3′-deoxythymidine for intravenous (IV) injections. Omnipaque® 300 (GE healthcare) was used for intraperitoneal (IP) injections. Positron emission tomography/computed tomography (PET/CT) scans were acquired on a Siemens Biograph® equipped with point spread function reconstruction. The optimal concentration and injection schedule of IP contrast agent was studied in 12 mice. The impact of IP contrast media on PET quantitative accuracy was investigated by phantom studies and by imaging six mice before and after IP injection of Omnipaque®. The impact of a dual contrast media protocol on tumor delineation and quantitation was evaluated in 15 tumor-bearing mice using ex vivo counting as the reference.
Results
The optimal sequence was a mixture of tracer plus IV contrast agent followed by 1 mL of IP contrast agent (20 mg iodine/mL) administered 10 min before PET/CT acquisition. Phantom studies showed that the use of a 20-mg iodine/mL concentration of Omnipaque® led to a 4.8% overestimation of radioactivity concentration, as compared to saline. This was confirmed by animal studies that demonstrated a 4.3% overestimation. Tumor detection was excellent and correlation between PET/CT quantitative data and ex vivo counting was good (r 2 = 0.91, slope = 0.7).
Conclusions
A dual radiologic contrast protocol is useful in PET/CT scanning of mice bearing abdominal tumors. Contrast agents used in this manner lead to a small but acceptable overestimation of quantitative PET data.






Similar content being viewed by others
References
Aqeilan RI, Hagan JP, Aqeilan HA, Pichiorri F, Fong LY, Croce CM (2007) Inactivation of the Wwox gene accelerates forestomach tumor progression in vivo. Cancer Res 67(12):5606–5610
Fujishita T, Doi Y, Sonoshita M et al (2004) Development of spontaneous tumours and intestinal lesions in Fhit gene knockout mice. Br J Cancer 91(8):1571–1574
Kizaka-Kondoh S, Itasaka S, Zeng L et al (2009) Selective killing of hypoxia-inducible factor-1-active cells improves survival in a mouse model of invasive and metastatic pancreatic cancer. Clin Cancer Res 15:3433–3441
Yi XF, Yuan ST, Lu LJ, Ding J, Feng YJ (2005) A clinically relevant orthotopic implantation nude mouse model of human epithelial ovarian cancer—based on consecutive observation. Int J Gynecol Cancer 15(5):850–855
Choquet P, Calon A, Breton E et al (2007) Multiple-contrast X-ray micro-CT visualization of colon malformations and tumours in situ in living mice. C R Biol 330(11):821–827
Graham KC, Detombe SA, MacKenzie LT et al (2008) Contrast-enhanced microcomputed tomography using intraperitoneal contrast injection for the assessment of tumor-burden in liver metastasis models. Invest Radiol 43(7):488–495
Willekens I, Lahoutte T, Buls N et al (2009) Time-course of contrast enhancement in spleen and liver with Exia 160, Fenestra LC, and VC. Mol Imaging Biol 11(2):128–135
Almajdub M, Nejjari M, Poncet G et al (2007) In vivo high-resolution X-ray microtomography for liver and spleen tumor assessment in mice. Contrast Media Mol Imaging 2(2):88–93
Suckow CE, Stout DB (2008) MicroCT liver contrast agent enhancement over time, dose, and mouse strain. Mol Imaging Biol 10(2):114–120
Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T (2005) Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med 11(1):63–70
Pichler BJ, Wehrl HF, Judenhofer MS (2008) Latest advances in molecular imaging instrumentation. J Nucl Med 49(Suppl 2):5S–23S
` Antoch G, Jentzen W, Freudenberg LS et al (2003) Effect of oral contrast agents on computed tomography-based positron emission tomography attenuation correction in dual-modality positron emission tomography/computed tomography imaging. Invest Radiol 38(12):784–789
Serdons K, Verbruggen A, Bormans G (2008) The presence of ethanol in radiopharmaceutical injections. J Nucl Med 49(12):2071
Cohade C, Osman M, Nakamoto Y et al (2003) Initial experience with oral contrast in PET/CT: phantom and clinical studies. J Nucl Med 44(3):412–416
Dizendorf E, Hany TF, Buck A, von Schulthess GK, Burger C (2003) Cause and magnitude of the error induced by oral CT contrast agent in CT-based attenuation correction of PET emission studies. J Nucl Med 44(5):732–738
Aide N, Desmonts C, Beauregard JM et al (2010) High throughput static and dynamic small animal imaging using clinical PET/CT: potential preclinical applications. Eur J Nucl Med Mol Imaging 37:991–1001
Denoyer D, Greguric I, Roselt P et al (2010) High-contrast PET of melanoma using (18)F-MEL050, a selective probe for melanin with predominantly renal clearance. J Nucl Med 51(3):441–447
Acknowledgments
This work was supported and by a Fellowship from the Fondation de France, and by a grant from the French Ligue contre le cancer, Comité du Calvados. The authors thank Emily Hong, Jason Callahan, David Binns and Mark Scalzo for their help during animals PET acquisitions and ex vivo counting and the animal technologists and research assistants (Kerry Ardley, Rachel Walker, Susan Jackson, Jeannette Valentan, Ekaterina Bogatyreva and Laura Kirby) from the Centre for Molecular Imaging for animal care and tracer injections. Dr. Aide is indebted to Dr. Lerouge for her continuous support during this work.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
A dual radiologic contrast protocol, which includes a mixture of tracer plus long-lasting intravenous contrast agent followed by intraperitoneal contrast agent, is described that provides better anatomical separation and delineation of abdominal tumors for PET/CT scanning of mice.
Rights and permissions
About this article
Cite this article
Aide, N., Kinross, K., Beauregard, JM. et al. A Dual Radiologic Contrast Agent Protocol for 18F-FDG and 18F-FLT PET/CT Imaging of Mice Bearing Abdominal Tumors. Mol Imaging Biol 13, 518–525 (2011). https://doi.org/10.1007/s11307-010-0378-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0378-x