Abstract
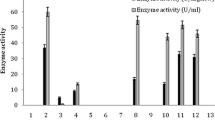
Bacillus megaterium F-8 exhibited an intracellular acetamide hydrolyzing activity (AHA) when cultivated in modified nutrient broth with 3 % tryptone, 1.5 % yeast extract, and 0.5 % sodium chloride, at pH 7.2, 45 °C for 24 h. Maximum AHA was recorded in the culture containing 0.1 M of sodium phosphate buffer, (pH 7.5) at 45 °C for 20 min with 0.2 % of acetonitrile and resting cells of B. megaterium F-8 equivalent to 0.2 ml culture broth. This activity was stable up to 55 °C and was completely inactivated at or above 60 °C. Maximum acyl transferase activity (ATA) was recorded in the reaction medium containing 0.1 M of potassium phosphate buffer, (pH 8.0) at 55 °C for 5 min with 0.85 mM of acetamide as acyl donor and hydroxylamine hydrochloride as acyl acceptor and resting cells of B. megaterium F-8 equivalent to 0.94 mg cells (dry weight basis). This activity was stable up to 60 °C and a rapid decline in enzyme activity was recorded above it. Under the optimized conditions, this organism hydrolyzed various nitriles and amides such as propionitrile, propionamide, caprolactam, acetamide, and acrylamide to corresponding acids. Acyl group transfer capability of this organism was used for the production of acetohydroxamic acid. ATA of B. megaterium F-8 showed broad substrate specificity such as for acetamide followed by propionamide, acrylamide, and lactamide. This amide hydrolyzing and amidotransferase activity of B. megaterium F-8 has potential applications in enzymatic synthesis of hydroxamic acids and bioremediation of nitriles and amides contaminated soil and water system.




Similar content being viewed by others
References
Andrade J, Frazao C (2007) Crystallization, diffraction data collection and preliminary crystallographic analysis of hexagonal crystals of Pseudomonas aeruginosa amidase. Acta Crystallogr Sect F: Struct Biol Cryst Commun 63:214–216. doi:10.1107/S1744309107005830
Banerjee A, Sharma R, Banerjee UC (2002) The nitrile-degrading enzymes: current status and future prospects. J Appl Microbiol and Biotechnol 17:133–147. doi:10.1007/s00253-002-1062-0
Bhalla TC, Kumar J, Kumar H, Agrawal HO (1997) Amidase production by Rhodococcus sp. NHB-2. Nat Acad Sci Lett 20:139–142
Bollag DM, Rozycki MD, Edelstein SJ (1996) Preparation for protein isolation. In: Protein Methods, 2nd edn. Wiley-Liss, Inc., New York, pp 1–27
Brammar WJ, Clarke PH (1964) Induction and repression of Pseudomonas aeruginosa amidase. J Gen Microbiol 37:307–319. doi:10.1099/00221287-37-3-307
Chand D, Kumar H, Sankhian U, Kumar D, Vitzthum F, Bhalla TC (2004) Treatment of simulated wastewater containing toxic amides by immobilized Rhodococcus rhodochrous NHB-2 using a highly compact 5-stage plug flow reactor. World Journal of Microbiology & Biotechnology 20:679–686. doi:10.1007/s11274-004-2158-8
Chapatwala KD, Babu GRV, Dudley C, Williams R, Aremu K (1993) Degradative capability of Pseudomonas putida on acetonitrile. Appl Biochem Biotechnol 39:655–666. doi:10.1007/BF02919026
Cramp R, Gilmour M, Cowan DA (1997) Novel thermophilic bacteria producing nitrile-degrading enzymes. Microbiology 143:2313–2320. doi:10.1099/00221287-143-7-2313
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. doi:10.1038/384083a0
Dhillon JK, Shivaraman N (1995) Biodegradation of cyanide compounds by a Pseudomonas species (S1). Can J Microbiol 45:201–208, PMID: 10408092
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159. doi:10.1136/jcp.13.2.156
Fournand D, Bigey F, Ratomahenina R, Annaud A, Galzy P (1997a) Biocatalyst improvement for the production of short-chain hydroxamic acids. Enzyme and Microbial Technol 20:424–431. doi:10.1016/S0141-0229(96)00170-6
Fournand D, Pirat JL, Bigey F, Arnaud A, Galzy P (1997b) Spectrophotometric assay of aliphatic monohydroxamic acids and α, β and γ aminohydroxamic acids in aqueous medium. Anal Chim Acta 353:359–366. doi:10.1021/jf950360j
Fournand D, Arnaud A, Galzy P (1998a) Studies of the acyl transfer activity of a recombinant amidase overproduced in an E. coli strain. Application for short chain hydroxamic acid and acid hydrazide synthesis. J Mol Catal B4:77–90. doi:10.1016/S1381-1177(97)00024-6
Fournand D, Bigey F, Arnaud A (1998b) Acyl transfer activity of an amidase from Rhodococcus sp. strain R312: formation of a wide range of hydroxamic acids. Appl Environ Microbiol 64:2844–2852, PMCID: PMC106781
Fournand D, Vaysse L, Dubreucq E, Annaud, Galzy P (1998c) Monohydroxamic acid biosynthesis. J Mol Catalysis B: Enzymatic 5:207–211. doi:10.1016/S1381-1177(98)00036-8
Graham D, Pereira R, Barfield D, Cowan D (2000) Nitrile biotransformations using free and immobilized cells of thermophilic Bacillus spp. Enzyme Microb Tech 26:368–373. doi:10.1016/S0141-0229(99)00169-6
Heiter HI, Ryles RG (1992) European patent 0514648:B1
Kelly M, Clarke PH (1962) An inducible amidase produced by a strain of Pseudomonas aeruginosa. J Gen Microbiol 27:305–316. doi:10.1099/00221287-27-2-305
Kobayashi M, Izui H, Nagasawa T, Yamada H (1993) Nitrilase biosynthesis of the plant hormone indole-3-acetic acid from indole acetonitrile: cloning of the Alcaligenes gene and site-directed mutagenesis of cysteine residues. PNAS USA 90:247–251. doi:10.1073/pnas.90.1.247
Kobayashi M, Shimizu S (1998) Metalloenzyme nitrile hydratase: structure, regulation and application to biotechnology. Nature Biotechnol 16:733–736. doi:10.1038/nbt0898-733
Kumar H, Prasad S, Raj J, Bhalla TC (2005) Constitutive acetonitrile hydrolyzing activity of Nocardia globerula NHB-2: Optimization of production and reaction conditions. Ind J Exp Biol 44:240–245. doi:10.1007/s10295-010-0902-7
Nawaz MS, Khan AA, Bhattacharayya D, Siitonen PH, Cerniglia CE (1996) Physical, biochemical and immunological characterization of a thermostable amidase from Klebsiella pneumoniae NCTR 1. J Bacteriol 178:2397–2401, PMID: 8636044
Pal A, Samanta TB (1999) β-Lactamase-free penicillin amidase from Alcaligenes sp.: isolation strategy, strain characteristics, and enzyme immobilization. Curr Microbiol 39:244–248. doi:10.1007/s002849900453
Patricelli MP, Cravatt BF (2000) Clarifying the catalytic roles of conserved residues in the amidase signature family. J Biol Chem 275:19177–19184. doi:10.1074/jbc
Brandao PF, Bull AT, Clapp JP (2003) Diversity of nitrile hydratase and amidase enzyme genes in Rhodococcus erythropolis recovered from geographically distinct habitats. Appl Environ Microbiol 69(10):5754–5766. doi:10.1128/AEM.69.10.5754-5766.2003
Ramakrishna C, Dave H, Ravindranathan M (1999) Microbial metabolism of nitriles and its biotechnological potential. J Sci Ind Res 58:925–947
Rezende R, Dias J (2003) The use of acetonitrile as the sole nitrogen and carbon source by Geotrichum sp. Jr1. Braz J Microbiol 34:117–120. doi:10.1590/S1517-83822004000100019
Sogani M, Mathur N, Bhatnagar P, Sharma P (2011) Constitutive acetonitrile hydrolyzing activity of Nocardia globerula NHB-2: biotransformation of amide using Bacillus sp.: isolation strategy, strain characteristics and enzyme immobilization. Ind J Env Sci technol. doi:10.1007/s13762-011-0005-7
Taylor SK, Chmiel NH, Simons LJ, Vyvyan JR (1996) Conversion of hydroxy nitriles to lactones using Rhodococcus rhodochrous whole cells. J Org Chem 61:9084–9085. doi:10.1021/jo9616623
Thalenfeld B, Grossowic ZN (1976) Regulatory properties of an inducible aliphatic amidase in a thermophilic bacillus. J Gen Microbiol 94:131–141. doi:10.1099/00221287-94-1-55
Thiėry AM, Maestracci A, Arnaud P, Nicolas M (1986a) Purification and properties of an acrylamide amidohydrolase (EC 3.5.1.4) with a wide activity spectrum from Brevibacterium sp. R312. J Basic Microbiol 26:299–311. doi:10.1002/jobm.3620260512
Thiėry A, Maestracci M, Arnaud A, Galzy P (1986b) Acyltransferase activity of the wide spectrum amidase of Brevibacterium sp. R312. J Gen Microbiol 132:2205–2208. doi:10.1099/00221287-132-8-2205
Tinggang L, Junxin L, Renbi B, Dieudonne G, Fook-Sin W (2007) Biodegradation of organonitriles by adapted activated sludge consortium with acetonitrile-degrading microorganisms. Water Res 41:3465–3473. doi:10.1016/j.watres.2007.04.033
Wang J, Long MC, Zhang ZJ (2008) Removal of organic compounds during treating printing and dyeing wastewater of different process units. Chemosphere 71:195–202. doi:10.1016/j, PMID: 17997469
Acknowledgments
We gratefully acknowledge the support and generosity of Director Mr. Arpit Agarwal, Jaipur Engineering College and Research Centre and the JECRC Foundation for providing necessary research facilities to carry out this investigation. The research for this paper was financially supported by Department of Science and Technology (DST), Government of India, vide their sanction no. 100/ (IFD)/1899/2012-13. We thank Parul Sharma, Anjita Chadda, and Tovindra Sahu for the assistance in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Rights and permissions
About this article
Cite this article
Sogani, M., Bakre, P.P., Mathur, N. et al. Acetamide hydrolyzing activity of Bacillus megaterium F-8 with bioremediation potential: Optimization of production and reaction conditions.. Environ Sci Pollut Res 21, 8822–8830 (2014). https://doi.org/10.1007/s11356-014-2818-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2818-7