Abstract
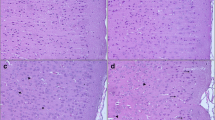
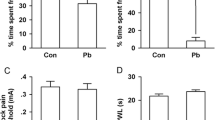
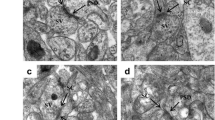
Lead (Pb) is a widespread environmental heavy metal that can damage the cerebral cortex and hippocampus, and reduce the learning and memory ability in humans and animals. In vivo and in vitro models of acute lead acetate exposure were established to further study the mechanism of neurons injury. In this study, 4-week-old female Kunming mice were randomly divided into four groups. Each group was treated with distilled water with different Pb concentrations (0, 2.4, 4.8 and 9.6 mM). Mice were killed, and brain tissues were collected to detect the changes in synaptic plasticity-related protein expression. Furthermore, Neuro-2A cells were treated with 0, 5, 25 and 50 μM lead acetate for 24 h to observe the changes in cell morphology and function. In in vivo experiment, results showed that the expression levels of cytoskeleton-associated and neural function-related proteins decreased in a dose-dependent manner in the mouse brain tissue. In in vitro experiment, compared with the control group, Pb treatment groups were observed with smaller and round cells, decreased cell density and number of synapses. In the Pb exposure group, the survival rate of nerve cells decreased evidently, and the permeability of the cell membrane was increased. Western blot results showed that the expression of cytoskeleton-associated and function-related proteins decreased gradually with increased Pb exposure dose. Confocal laser scanning microscopy results revealed the morphological and volumetric changes in Neuro-2A cells, and a dose-dependent reduction in the number of axon and dendrites. These results suggested that abnormal neural structures and inhibiting expression of synaptic plasticity-related proteins might be the possible mechanisms of Pb-induced mental retardation in human and animals, thereby laying a foundation for the molecular mechanism of Pb neurotoxicity.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Arslan A (2021) Extrasynaptic δ-subunit containing GABAA receptors. J Integr Neurosci 30;20(1):173–184. https://pubmed.ncbi.nlm.nih.gov/33834705/
Caldas D, Pestana IA, Almeida MG, Henry FC, Salomão MS, de Souza CM (2016) Risk of ingesting As, Cd, and Pb in animal products in north Rio de Janeiro state, Brazil. Chemosphere 164:508–515
Chen L, Chen H, Yao C, Chang C, Xia H, Zhang C, Zhou Y, Yao Q, Chen K (2015) The toxicity of NaF on BmN cells and a comparative proteomics approach to identify protein expression changes in cells under NaF-stress: impact of NaF on BmN cells. J Hazard Mater 286:624–31
Chen F, Zhou CC, Yang Y, Liu JW, Yan CH (2019) GM1 Ameliorates Lead-Induced Cognitive Deficits and Brain Damage Through Activating the SIRT1/CREB/BDNF Pathway in the Developing Male Rat Hippocampus. Biol Trace Elem Res 190(2):425–436
Chen L, Ning H, Yin Z, Song X, Feng Y, Qin H, Li Y, Wang J, Ge Y, Wang W (2017) The effects of fluoride on neuronal function occurs via cytoskeleton damage and decreased signal transmission. Chemosphere 185:589–594
Choi WS, Kim SJ, Kim JS (2011) Inorganic lead (Pb)- and mercury (Hg)-induced neuronal cell death involves cytoskeletal reorganization. Lab Anim Res 27(3):219–25
Dehmelt L, Halpain S (2004) The MAP2/Tau family of microtubule-associated proteins. Genome Biol 6(1):204
Ding JJ, Zou RX, He HM, Lou ZY, Xu Y, Wang HL (2018) Pb inhibits hippocampal synaptic transmission via cyclin-dependent kinase-5 dependent Synapsin 1 phosphorylation. Toxicol Lett 296:125–131. https://pubmed.ncbi.nlm.nih.gov/30121340/
dos Remedios C G, Chhabra D, Kekic M, Dedova I V, Tsubakihara M., Berry D A, Nosworthy N J (2003) Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev 83(2): 433–73. https://pubmed.ncbi.nlm.nih.gov/12663865/
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5(2):47–58
Gąssowska M, Baranowska-Bosiacka I, Moczydłowska J, Frontczak-Baniewicz M, Gewartowska M, Strużyńska L, Gutowska I, Chlubek D, Adamczyk A (2016a) Perinatal exposure to lead (Pb) induces ultrastructural and molecular alterations in synapses of rat offspring. Toxicology 373:13–29
Gąssowska M, Baranowska-Bosiacka I, Moczydłowska J, Tarnowski M, Pilutin A, Gutowska I, Strużyńska L, Chlubek D, Adamczyk A (2016b) Perinatal exposure to lead (Pb) promotes Tau phosphorylation in the rat brain in a GSK-3β and CDK5 dependent manner: relevance to neurological disorders. Toxicology 347–349:17–28
Ge Y, Chen L, Sun X, Yin Z, Song X, Li C, Liu J, An Z, Yang X, Ning H (2018a) Lead-induced changes of cytoskeletal protein is involved in the pathological basis in mice brain. Environ Sci Pollut Res Int 25(12):11746–11753
Ge Y, Chen L, Yin Z, Song X, Ruan T, Hua L, Liu J, Wang J, Ning H (2018b) Fluoride-induced alterations of synapse-related proteins in the cerebral cortex of ICR offspring mouse brain. Chemosphere 201:874–883
Ge Y, Song X, Chen L, Hu D, Hua L, Cui Y, Liu J, An Z, Yin Z, Ning H (2019) Cadmium induces actin cytoskeleton alterations and dysfunction in Neuro-2a cells. Environ Toxicol 34(4):469–475
Gulisano W, Maugeri D, Baltrons MA, Fà M, Amato A, Palmeri A, D'Adamio L, Grassi C, Devanand DP, Honig LS, Puzzo D, Arancio O (2018) Role of Amyloid-β and Tau Proteins in Alzheimer's Disease: Confuting the Amyloid Cascade. J Alzheimers Dis 64(s1): S611-S631. https://www.j-alz.com/manuscript-disclosures/17-9935
Gumy LF, Katrukha EA, Grigoriev I, Jaarsma D, Kapitein LC, Akhmanova A, Hoogenraad CC (2017) Map2 defines a pre-axonal filtering zone to regulate kif1-versus kif5-dependent cargo transport in sensory neurons. Neuron 94(2):347–362
Hayashi Y, Nishimune H, Hozumi K, Saga Y, Harada A, Yuzaki M, Iwatsubo T, Kopan R, Tomita T (2016) A novel non-canonical Notch signaling regulates expression of synaptic vesicle proteins in excitatory neurons. Sci Rep 6:23969
Ketschek A, Spillane M, Dun XP, Hardy H, Chilton J, Gallo G (2016) Drebrin coordinates the actin and microtubule cytoskeleton during the initiation of axon collateral branches. Dev Neurobiol 76(10):1092–110
Kumar D, Thakur MK (2017) Anxiety like behavior due to perinatal exposure to Bisphenol-A is associated with decrease in excitatory to inhibitory synaptic density of male mouse brain. Toxicology 378:107–113
Liu Y, Xu YF, Zhang L, Huang L, Yu P, Zhu H, Deng W, Qin C (2017a) Effective expression of Drebrin in hippocampus improves cognitive function and alleviates lesions of Alzheimer's disease in APP (swe)/PS1 (ΔE9) mice. CNS Neurosci Ther 23(7):590–604. https://pubmed.ncbi.nlm.nih.gov/28597477/
Liu Y, Xu Y, Zhang L, Huang L, Yu P, Zhu H, Deng W, Qin C (2017b) Down-Regulated Drebrin Aggravates Cognitive Impairments in a Mouse Model of Alzheimer’s Disease. Int J Mol Sci 18(4):800
Nascimento CRB, Risso WE, Martinez CBDR (2016) Lead accumulation and metallothionein content in female rats of different ages and generations after daily intake of Pb-contaminated food. Environ Toxicol Pharmacol 48:272–277
Neal AP, Guilarte TR (2010) Molecular neurobiology of lead (Pb (2+)): effects on synaptic function. Mol Neurobiol 42(3):151–60
Neelima A, Rajanna A, Bhanuprakash RG, Chetty CS, Suresh C (2017) Deleterious effects of combination of lead and β-amyloid peptides in inducing apoptosis and altering cell cycle in human neuroblastoma cells. Interdiscip Toxicol 10(3):93–98
Ning H, Li C, Yin Z, Hu D, Ge Y, Chen L (2021) Fluoride exposure decreased neurite formation on cerebral cortical neurons of SD rats in vitro. Environ Sci Pollut Res Int 28(37):50975–50982
Ohkawa N, Hashimoto K, Hino T, Migishima R, Yokoyama M, Kano M, Inokuchi K (2007) Motor discoordination of transgenic mice overexpressing a microtubule destabilizer, stathmin, specifically in Purkinje cells. Neurosci Res 59(1):93–100. https://pubmed.ncbi.nlm.nih.gov/17640754/
Paik NJ, Yang E (2014) Role of GABA plasticity in stroke recovery. Neural Regen Res 9(23):2026–8. https://pubmed.ncbi.nlm.nih.gov/25657711/
Ramírez Ortega D, González Esquivel DF, Blanco Ayala T, Pineda B, Gómez Manzo S, Marcial Quino J, Carrillo Mora P, Pérez de la Cruz V (2021) Cognitive impairment induced by lead exposure during lifespan: mechanisms of lead neurotoxicity. Toxics 9(2):23
Shirao T, González-Billault C (2013) Actin filaments and microtubules in dendritic spines. J Neurochem 126(2):155–64
Takizawa H, Hiroi N, Funahashi A (2012) Mathematical modeling of sustainable synaptogenesis by repetitive stimuli suggests signaling mechanisms in vivo. PLoS One 7(12):e51000
Tanabe K, Yamazaki H, Inaguma Y, Asada A, Kimura T, Takahashi J, Taoka M, Ohshima T, Furuichi T, Isobe T, Nagata K, Shirao T, Hisanaga S (2014) Phosphorylation of drebrin by cyclin-dependent kinase 5 and its role in neuronal migration. PLoS One 9(3):e92291
Verstraeten SV, Aimo L, Oteiza PI (2008) Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol 82(11):789–802
Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR (1998) Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 79(2):555–66. https://pubmed.ncbi.nlm.nih.gov/9463421/
Wagner PJ, Park HR, Wang Z, Kirchner R, Wei Y, Su L, Stanfield K, Guilarte TR, Wright RO, Christiani DC, Lu Q (2017) In vitro effects of lead on gene expression in neural stem cells and associations between up-regulated genes and cognitive scores in children. Environ Health Perspect 125(4):721–729
Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH (2008) Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28:3–9
Xiang Y, Niu Y, Xie Y, Chen S, Zhu F, Shen W, Zeng LH (2021) Inhibition of RhoA/Rho kinase signaling pathway by fasudil protects against kainic acid-induced neurite injury. Brain Behav 11(8): e2266. https://publons.com/publon/10.1002/brb3.2266
Yin Z, Hua L, Chen L, Hu D, Li J, An Z, Tian T, Ning H, Ge Y (2020) Bisphenol-A exposure induced neurotoxicity and associated with synapse and cytoskeleton in Neuro-2a cells. Toxicol In Vitro 67:104911
Yu H, Li T, Cui Y, Liao Y, Wang G, Gao L, Zhao F, Jin Y (2014) Effects of lead exposure on D-serine metabolism in the hippocampus of mice at the early developmental stages. Toxicology 325:189–199
Yu H, Liao Y, Li T, Cui Y, Wang G, Zhao F, Jin Y (2016) Alterations of synaptic proteins in the hippocampus of mouse offspring induced by developmental lead exposure. Mol Neurobiol 53(10):6786–6798
Zarin B, Eshraghi A, Zarifi F, Javanmard SH, Laher I, Amin B, Vaseghi G (2021) A review on the role of tau and stathmin in gastric cancer metastasis. Eur J Pharmacol 908:174312
Zhang XL, Guariglia SR, McGlothan JL, Stansfield KH, Stanton PK, Guilarte TR (2015) Presynaptic mechanisms of lead neurotoxicity: effects on vesicular release, vesicle clustering and mitochondria number. PLoS One 10(5):1–21
Zhao F, Liao Y, Tang H, Piao J, Wang G, Jin Y (2017) Effects of developmental arsenite exposure on hippocampal synapses in mouse offspring. Metallomics 9(10):1394–1412
Zhou C-C, Gao Z-Y, Wang J, Wu M-Q, Hu S, Chen F, Liu J-X, Pan H, Yan C-H (2018) Lead exposure induces Alzheimers’s disease (AD)-like pathology and disturbes cholesterol metabolism in the young rat brain. Toxicol Lett 296:173–183
Acknowledgements
This work was supported by the National Science Foundation of China (32002352), the Nature and Scientific Foundation of Henan Province in China (202300410165), the Key Research Projects in Colleges of Henan province (22A330001 and 22A230007) and the Scientific and Technological Foundation of Henan Province in China (212102110102).
Author information
Authors and Affiliations
Contributions
Lingli Chen contributed to design and data analysis, Yuye Liu wrote the manuscript, Penghuan Jia and Hongli Zhang carried out the experiments and collected the data, Zhihong Yin, Dongfang Hu and Hongmei Ning contributed to revise the manuscript, and Yaming Ge managed the project.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All of the procedures involving the cells and animals were approved by and performed according to the guidelines of the Animal Ethics Committee of the Henan Institute of Science and Technology.
All authors have read and approved this version of the article and consent to publish.
Consent for publication
Traditional publishing model.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Ludek Blaha
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, L., Liu, Y., Jia, P. et al. Acute lead acetate induces neurotoxicity through decreased synaptic plasticity-related protein expression and disordered dendritic formation in nerve cells. Environ Sci Pollut Res 29, 58927–58935 (2022). https://doi.org/10.1007/s11356-022-20051-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20051-1