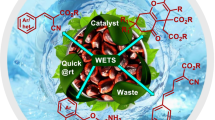
Abstract
A tremendous research has been appeared on Pd-catalyzed Suzuki–Miyaura cross-coupling (SMC) during the last four decades due to its high prominence in constructing biaryl motifs of several complexes as well as simple organic compounds of high biological and commercial significance. The use of organic solid waste–derived materials for SMC in benign solvents like water/aqueous media is a very good achievement in these cases. We report in this article the usability of water extract of Tamarindus indica seeds ash (WETS) as a renewable base and reaction medium for Pd(OAc)2-catalyzed SMC reaction at room temperature (RT). The WETS has been characterized using powder XRD, EDAX, SEM, and FTIR analysis. Furthermore, this process is highly environmentally beneficial by the waste repurposing to prominent chemical transformation along with the advantages such as ambient condition and avoids non-renewable chemicals like volatile organic solvents, ligands, promoters, and bases. Based on these merits and the quick reactions with high yields of products, this method can attain the interest of the scientific community in exploring the waste-derived ashes to significant chemical transformations.
Graphical abstract
Tamarindus indica seed ash extract for C–C coupling under added organics and volatile organic solvent-free conditions: a waste repurposing technique for Suzuki–Miyaura reaction




Similar content being viewed by others
Data Availability
The spectral data and the copies of spectra of the biaryls have been provided at supporting information, which can be accessed at online version of this article.
References
Ackermann L, Althammer A (2006) Air-stable PinP(O)H as preligand for palladium-catalyzed Kumada couplings of unactivated tosylates. Org Lett 8:3457–3460
Abdullah HSTSH, Asseri SNARM, Mohamad WNKW, Kan S-Y, Azmi AA, Julius FSY, Chia PW (2021) Green synthesis, characterization and applications of silver nanoparticle mediated by the aqueous extract of red onion peel. Environ Pollut 271:116295
Appa RM, Lakshmidevi J, Naidu BR, Venkateswarlu K (2021a) Pd-catalyzed oxidative homocoupling of arylboronic acids in WEPA: a sustainable access to symmetrical biaryls under added base and ligand-free ambient conditions. Mol Catal 501:111366
Appa RM, Raghavendra P, Lakshmidevi J, Naidu BR, Sarma LS, Venkateswarlu K (2021b) Structure controlled Au@Pd NPs/rGO as robust heterogeneous catalyst for Suzuki coupling in biowaste-derived water extract of pomegranate ash. Appl Organomet Chem 35:e6188
Ardhapure AV, Cholap A, Schulzke C, Maiti D, Kapdi AR (2018) Stille cross-coupling reaction: early years to the current state of the art, in palladium-catalyzed modification of nucleosides, nucleotides and oligonucleotides. Elsevier, pp. 19–36
Baran NY, Baran T, Menteş A (2017) Fabrication and application of cellulose Schiff base supported Pd(II) catalyst for fast and simple synthesis of biaryls via Suzuki coupling reaction. Appl Catal A 531:36–44
Beaven GH, de la Mare PBD, Hassan M, Johnson EA, Klassen NV (1961) 537. The kinetics and mechanisms of aromatic halogen substitution. Part X. Products in the chlorination of biphenyl in acetic acid. J Chem Soc 2749–2755
Bergstrom FW, McAllister SH (1930) The preparation of 2-alkyl and 3-aryl pyridines and quinolones by the Grignard reaction (preliminary). J Am Chem Soc 52:2845–2849
Blettner CG, König WA, Stenzel W, Schotten T (1999) Microwave-assisted aqueous Suzuki cross-coupling reactions. J Org Chem 64:3885–3890
Boruah PR, Ali AA, Saikia B, Sarma D (2015) A novel green protocol for ligand free Suzuki-Miyaura cross-coupling reactions in WEB at room temperature. Green Chem 17:1442–1445
Boztepe C, Künkül A, Yaşar S, Gürbüz N (2018) Heterogenization of homogeneous NHC-Pd-pyridine catalysts and investigation of their catalytic activities in Suzuki-Miyaura coupling reactions. J Organomet Chem 872:123–134
Braga AAC, Morgon NH, Ujaque G, Maseras F (2005) Computational characterization of the role of the base in the Suzuki−Miyaura cross-coupling reaction. J Am Chem Soc 127:9298–9307
Cataldo F (ed) (2005) Polyynes: synthesis properties, and applications. CRC Press/Taylor & Francis, Boca Raton, FL
Catellani M, Motti E, Ca ND (2008) Catalytic sequential reactions involving palladacycle-directed aryl coupling steps. Acc Chem Res 41:1512–1522
Chen C, Liu P, Luo M, Zeng X (2018) Kumada arylation of secondary amides enabled by chromium catalysis for unsymmetric ketone synthesis under mild conditions. ACS Catal 8:5864–5868
Chia PW, Chee PS, Asseri SNARM, Yong FSJ, Kan SY (2018) Synthesis of 2-aminobiaryl derivatives promoted by water extract of onion peel ash. Malays J Anal Sci 22:742–749
Chia PW, Chee PS, Aziz MH, Radzi SAM, Yong FSJ, Kan SY (2019a) Water extract of onion peel ash: an efficient green catalytic system for the synthesis of isoindoline-1,3-dione derivatives. Malays J Anal Sci 23:23–30
Chia PW, Chee PS, Mazlan NW, Yong FSJ, Rozaini MZH, Kan S-Y (2020) Acetylation of alcohols and amines catalyzed by onion peel ash under a base- and solvent-free condition. Songklanakarin J Sci Technol 42:602–607
Chia PW, Lim BS, Tan KC, Yong FSJ, Kan SY (2019b) Water Extract of Onion Peel for the Synthesis of Bisindolylmethanes. J King Saud Univ Sci 31:642–647
Cordovilla C, Bartolomé C, Martínez-Ilarduya JM, Espinet P (2015) The Stille reaction, 38 years later. ACS Catal 5:3040–3053
Crowley JD, Goldup SM, Lee A-L, Leigh DA, McBurney RT (2009) Active metal template synthesis of rotaxanes, catenanes and molecular shuttles. Chem Soc Rev 38:1530–1541
de Rivera FG, Angurell I, Rossell MD, Erni R, Llorca J, Divins NJ, Muller G, Seco M, Rossell O (2013) A general approach to fabricate Fe3O4 nanoparticles decorated with Pd, Au, and Rh: magnetically recoverable and reusable catalysts for Suzuki C-C cross-coupling reactions, hydrogenation, and sequential reactions. Chem Eur J 19:11963–11974
Decottignies A, Fihri A, Azemar G, Djedaini-Pilard F, Len C (2013) Ligandless Suzuki-Miyaura reaction in neat water with or without native β-cyclodextrin as additive. Catal Commun 32:101–107
Diederich F, Stang PJ, Tykwinski RR (2005) Acetylene chemistry: chemistry, biology and material science. Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim
Effenberger F, Koch M, Streicher W (1991) Nucleophile substitution von nitrit in nitrobenzolen, nitrobiphenylen und nitronaphthalinen. Chem Ber 124:163–173
Fan M, Wu H, Shi M, Zhang P, Jiang P (2019) Well-dispersive K2O–KCl alkaline catalyst derived from waste banana peel for biodiesel synthesis. Green Energ Environ 4:322–327
Felpin F- X, Fouquet E, Zakri C 2009 Improved Suzuki-Miyaura reactions of aryldiazonium salts with boronic acids by tuning palladium on charcoal catalyst properties Adv Synth Catal 351 649 655
Fırıncı R, Günay ME, Gökçe AG (2017) Synthesis, characterization and catalytic activity in Suzuki-Miyaura coupling of palladacycle complexes with n-butyl-substituted N-heterocyclic carbene ligands. Appl Organomet Chem 32:e4109
Gavryushin A, Kofink C, Manolikakes G, Knochel P (2006) An efficient Negishi cross-coupling reaction catalyzed by nickel(II) and diethyl phosphite. Tetrahedron 62:7521–7533
Gennaro A, Isse AA, Bianchi CL, Mussini PR, Rossi M (2009) Is glassy carbon a really inert electrode material for the reduction of carbon–halogen bonds? Electrochem Commun 11:1932–1935
Haas D, Hammann JM, Greiner R, Knochel P (2016) Recent developments in Negishi cross-coupling reactions. ACS Catal 6:1540–1552
Hervé G, Sartori G, Enderlin G, Mackenzie G, Len C (2014) Palladium-catalyzed Suzuki reaction in aqueous solvents applied to unprotected nucleosides and nucleotides. RSC Adv 4:18558–18594
Ikram M, Inayat T, Haider A, Hamid AU, Haider J, Nabgan W, Saeed A, Shahbaz A, Hayat S, Ain KU, Butt AR (2021) Graphene oxide-doped MgO nanostructures for highly efficient dye degradation and bactericidal action. Nanoscale Res Lett 16:56
Jabbari A, Tahmasbi B, Nikoorazm M, Ghorbani-Choghamarani A (2018) A new Pd-Schiff-base complex on boehmite nanoparticles: its application in Suzuki reaction and synthesis of tetrazoles. Appl Organomet Chem 32:e4295
Jin L, Wei W, Sun N, Hu B, Shen Z, Hu X (2018) Unsymmetrical CNN-palladacycles with geometry-constrained iminopyridyl ligands: an efficient precatalyst in Suzuki coupling for accessing 1,1-diarylalkanes from secondary benzylic bromides. Org Chem Front 5:2484–2491
Kosaka K, Uchida T, Mikami K, Ohta Y, Yokozawa T (2018) Am Phos Pd-catalyzed Suzuki-Miyaura catalyst-transfer condensation polymerization: narrower dispersity by mixing the catalyst and base prior to polymerization. Macromolecules 51:364–369
Kostas ID, Coutsolelos AG, Charalambidis G, Skondra A (2007) The first use of porphyrins as catalysts in cross-coupling reactions: a water-soluble palladium complex with a porphyrin ligand as an efficient catalyst precursor for the Suzuki-Miyaura reaction in aqueous media under aerobic conditions. Tetrahedron Lett 48:6688–6691
Lakshmidevi J, Naidu BR, Venkateswarlu K (2022) A rapid-room temperature synthesis of α-cyanoacrylates, α-cyanoacrylonitriles and 4H-pyrans using water extract of pomegranate ash as catalytic media. Sustain Chem Pharm 25:100610
Lakshmidevi J, Vakati V, Naidu BR, Raghavender M, Rao KSVK, Venkateswarlu K (2021) Pd(5%)-KIT-6, Pd(5%)-SBA-15 and Pd(5%)-SBA-16 catalysts in water extract of pomegranate ash: a case study in heterogenization of Suzuki-Miyaura reaction under external base and ligand free conditions. Sustain Chem Pharm 19:100371
Lee YM, Lim C, Lee HS, Shin YK, Shin K-O, Lee YM, Kim S (2013) Synthesis and biological evaluation of a polyyne-containing sphingoid base probe as a chemical tool. Bioconjugate Chem 24:1324–1331
Lei P, Meng G, Szostak M (2017) General method for the Suzuki-Miyaura cross-coupling of amides using commercially available, air- and moisture-stable palladium/NHC (NHC = N-heterocyclic carbene) complexes. ACS Catal 7:1960–1965
Leowanawat P, Zhang N, Resmerita A-M, Rosen BM, Percec V (2011) Ni(COD)2/PCy3 catalyzed cross-coupling of aryl and heteroaryl neopentylglycolboronates with aryl and heteroaryl mesylates and sulfamates in THF at room temperature. J Org Chem 76:9946–9955
Li G, Lei P, Szostak M, Casals-Cruañas E, Poater A, Cavallo L, Nolan SP (2018) Mechanistic study of Suzuki-Miyaura cross-coupling reactions of amides mediated by [Pd(NHC)(allyl)Cl] Precatalysts. ChemCatChem 10:3096–3106
Li Z, Gelbaum C, Heaner WL IV, Fish J, Jaganathan A, Holden B, Pollet P, Liotta CL (2016) Palladium-catalyzed Suzuki reactions in water with no added ligand: effects of reaction scale, temperature, pH of aqueous phase, and substrate structure. Org Process Res Dev 20:1489–1499
Mao J, Gu Q, Gregory GH (2015) Revisiting the hydrogen storage behavior of the Na-O-H system. Materials 8:2191–2203
Mino T, Shirae Y, Sakamoto M, Fujita T (2005) C2-Symmetric bis-hydrazones as ligands in the asymmetric Suzuki−Miyaura cross-coupling. J Org Chem 70:2191–2194
Miyaura N (2002) Cross-coupling reaction of organoboron compounds via base-assisted transmetalation to palladium(II) complexes. J Organomet Chem 653:54–57
Naidu BR, Lakshmidevi J, Naik BS, Venkateswarlu K (2021) Water extract of pomegranate ash as waste-originated biorenewable catalyst for the novel synthesis of chiral tert-butanesulfinyl aldimines in water. Mol Catal 511:111719
Nakao Y, Hiyama T (2011) Silicon-based cross-coupling reaction: an environmentally benign version. Chem Soc Rev 40:4893–4901
Nakayama S, Uto Y, Tanimoto K, Okuno Y, Sasaki Y, Nagasawa H, Nakata E, Arai K, Momose K, Fujita T, Hashimoto T, Okamoto Y, Asakawa Y, Goto S, Hori H (2008) TX-2152: a conformationally rigid and electron-rich diyne analogue of FTY720 with in vivo antiangiogenic activity. Bioorg Med Chem 16:7705–7714
Negishi E-i (2011) Magical power of transition metals: past, present, and future (Nobel Lecture). Angew Chem Int Ed 50:6738–6764
Patel DC, Breitbach ZS, Woods RM, Lim Y, Wang A, Foss FW Jr, Armstrong DW (2016) Gram scale conversion of R-BINAM to R-NOBIN. J Org Chem 81:1295–1299
Peixoto MLB, Silva CHL, Godoi M (2022) Generation of new carbon–carbon and carbon–heteroatom bonds mediated by agro-waste extracts: a review. Environ Chem Lett 20:841–873
Peng Y-Y, Liu J, Lei X, Yin Z (2010) Room-temperature highly efficient Suzuki-Miyaura reactions in water in the presence of Stilbazo. Green Chem 12:1072–1075
Polshettiwar V, Decottignies A, Len C, Fihri A (2010) Suzuki-Miyaura cross-coupling reactions in aqueous media: green and sustainable syntheses of biaryls. Chemsuschem 3:502–522
Rao KU, Appa RM, Lakshmidevi J, Vijitha R, Rao KSVK, Narasimhulu M, Venkateswarlu K (2017) C(sp2)−C(sp2) coupling in water: palladium(II) complexes of N-pincer tetradentate porphyrins as effective catalysts. Asian J Org Chem 6:751–757
Rao KU, Venkateswarlu K (2018) PdII-porphyrin complexes – the first use as safer and efficient catalysts for Miyaura borylation. Synlett 29:1055–1060
Seganish WM, DeShong P (2004) Application of directed orthometalation toward the synthesis of aryl siloxanes. J Org Chem 69:6790–6795
Saha D, Chattopadhyay K, Ranu BC (2009) Aerobic ligand-free Suzuki coupling catalyzed by in situ-generated palladium nanoparticles in water. Tetrahedron Lett 50:1003–1006
Sarina S, Zhu H, Jaatinen E, Xiao Q, Liu H, Jia J, Chen C, Zhao J (2013) Enhancing catalytic performance of palladium in gold and palladium alloy nanoparticles for organic synthesis reactions through visible light irradiation at ambient temperatures. J Am Chem Soc 135:5793–5801
Sawai K, Tatumi R, Nakahodo T (2008) Fujihara H (2008) Asymmetric Suzuki-Miyaura coupling reactions catalyzed by chiral palladium nanoparticles at room temperature. Angew Chem Int Ed 47:6917–6919
Schweizer S, Becht J-M, Le Drian C (2007) Highly efficient and reusable supported Pd catalysts for Suzuki−Miyaura reactions of aryl chlorides. Org Lett 9:3777–3780
Seo K-B, Lee I-H, Lee J, Choi I, Choi T-L (2018) A rational design of highly controlled Suzuki-Miyaura catalyst-transfer polycondensation for precision synthesis of polythiophenes and their block copolymers: marriage of palladacycle precatalysts with MIDA-boronates. J Am Chem Soc 140:4335–4343
Siemsen P, Livingston RC, Diederich F (2000) Acetylenic coupling: a powerful tool in molecular construction. Angew Chem Int Ed 39:2632–2657
Spivey AC, Diaper CM, Adams H, Rudge AJ (2000) A new germanium-based linker for solid phase synthesis of aromatics: synthesis of a pyrazole library. J Org Chem 65:5253–5263
Suzuki A (2011) Cross-coupling reactions of organoboranes: an easy way to construct C-C bonds (Nobel Lecture). Angew Chem Int Ed 50:6722–6737
Tay J-H, Arguelles AJ, Nagorny P (2015) Direct interconversion of BINOL and H8-BINOL-based chiral Bronsted acids using single-step red/ox manipulations. Org Lett 17:3774–3777
Uemura T, Zhang XY, Matsumura K, Sayo N, Kumobayashi H, Ohta T, Nozaki K, Takaya H (1996) Highly efficient enantioselective synthesis of optically active carboxylic acids by Ru(OCOCH3)2[(S)-H8-BINAP]. J Org Chem 61:5510–5516
Venkateswarlu K (2021) Ashes from organic waste as reagents in synthetic chemistry: a review. Environ Chem Let 19:3887–3950
Wang H, Yang J (2017) Piperazine- and DABCO-bridged dinuclear N-heterocyclic carbene palladium complexes: synthesis, structure and application to Hiyama coupling reaction. Appl Organomet Chem 31:e3543
Wong W-Y (2005) Recent advances in luminescent transition metal polyyne polymers. J Inorg Organomet Polym Mater 15:197–219
Wu L, Li Z-W, Zhang F, He Y-M, Fan Q-H (2008) Air-stable and highly active dendritic phosphine oxide-stabilized palladium nanoparticles: preparation, characterization and applications in the carbon-carbon bond formation and hydrogenation reactions. Adv Synth Catal 350:846–862
Wu Z, Li Y, Gao L, Wang S, Fu G (2016) Synthesis of Na-doped ZnO hollow spheres with improved photocatalytic activity for hydrogen production. Dalton Trans 45:11145–11149
Xiao Q, Sarina S, Jaatinen E, Jia J, Arnold DP, Liu H, Zhu H (2014) Efficient photocatalytic Suzuki cross-coupling reactions on Au–Pd alloy nanoparticles under visible light irradiation. Green Chem 16:4272–4285
Xie L-G, Wang Z-X (2010) Nickel-catalyzed cross-coupling of non-activated or functionalized aryl halides with aryl Grignard reagents. Chem Eur J 16:10332–10336
Yap YH, Azmi AA, Mohd NK, Yong FSJ, Kan SY, Thirmizir MZA, Chia PW (2020) Green synthesis of silver nanoparticle using water extract of onion peel and application in the acetylation reaction. Arab J Sci Eng 45:4797–4807
Funding
KSK: University Grants Commission (UGC), New Delhi, for faculty position under FRP. OU-DST-PURSE-II and UGC-UPE-FAR funded this project.
Author information
Authors and Affiliations
Contributions
KSK and KV conceived and designed the research. BB, MR, and BRN conducted the experiments. All the authors analyzed the data and drafted, edited, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This manuscript does not contain the studies with human participants or animals performed by any of the others.
Consent of participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhaskar, B., Raghavender, M., Ramesh Naidu, B. et al. Tamarindus indica seed ash extract for C–C coupling under added organics and volatile organic solvent-free conditions: a waste repurposing technique for Suzuki–Miyaura reaction. Environ Sci Pollut Res 30, 71430–71438 (2023). https://doi.org/10.1007/s11356-022-20407-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20407-7