Abstract
Background, aim, and scope
In the soil environment, polycyclic aromatic hydrocarbons (PAHs) and heavy metals (HMs) are of great environmental and human health concerns due to their widespread occurrence, persistence, and carcinogenic properties. Bioremediation of contaminated soil is a cost-effective, environmentally friendly, and publicly acceptable approach to address the removal of environmental contaminants. However, bioremediation of contaminants depends on plant–microbe interactions in the rhizosphere. The microorganisms that can mineralize various PAHs have PAH dioxygenase genes like nahAc, phnAc, and pdo1. To understand the fate of pyrene in rhizospheric and non-rhizospheric soils in the presence or absence of Pb, pyrene biodegradation, bacterial community structure, and dioxygenase genes were investigated in a pot experiment.
Methods
Soil was amended with Pb (a representative heavy metal), pyrene, and a Pb/pyrene mixture. After 8 weeks of aging, one set of pot microcosms was cultivated with rye grass (Lolium multiflorum L) seedlings, while another set was not cultivated for the purpose of comparing rhizosphere and non-rhizosphere pyrene degradation. Pyrene was extracted from freeze-dried soil and plant samples using a Soxhlet extraction method and the extracts were dried to 1 mL under gentle nitrogen flow and analyzed using gas chromatograph mass spectrometry (Agilent 6890, USA). Soil DNA was extracted from triplicate samples and the DGGE was performed using a Bio-Rad Dcode™ Universal Mutation Detection System (Bio-Rad, USA). PAH dioxygenase genes, including nahAc, phnAc, and pdo1, were detected using PCR amplification. Similarly, pyrene degraders were also investigated using plate counting technique.
Results
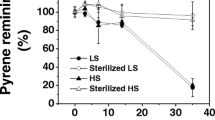
Biodegradation rates recorded over an 18-week period showed that rye grass promoted significant (P < 0.05) pyrene degradation. Pyrene removal efficiency from rhizospheric soils was 59.1 ± 2.1% and 68.7 ± 2.3% in pyrene- and Pb/pyrene-amended soils, respectively. The results indicate that pyrene dissipation was significantly (P < 0.05) higher in Pb/pyrene-amended soils than only-pyrene-amended soils. The plant growth promoted the degradation of pyrene and accounted for 12.1% to 17.0% of dissipation enhancement in the rhizospheric soils. In this study, the DGGE profiles revealed a shift in soil bacterial community structure in all amended soils, with a higher number and greater complexity of banding patterns in Pb/pyrene-amended samples than in either Pb- or pyrene-amended samples. In the control and Pb-amended soil, pdo1 and nahAc genes were not detected throughout the incubation period but were detected in the pyrene- and Pb/pyrene-amended soils. However, phnAc genes were not detected in either amended or non-amended soils throughout the incubation period. The addition of pyrene had a dramatic effect on the number of pyrene degraders.
Discussion
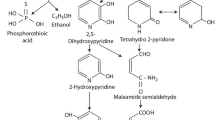
Plants contribute to the degradation of PAHs by increasing the size of microbial population, promoting microbial activity, and modifying microbial community diversity in the rhizosphere. In this study, the presence of plants significantly promoted the degradation of pyrene in the soil due to enhanced bacterial community size and increased the number of pyrene degraders. Similarly, the results of this study have also clearly shown that pyrene remaining in soils only accounted for about 1/3 of the total pyrene addition, suggesting that most of the pyrene added could be removed by plant and/or microbial degradation. HMs and PAHs interaction towards degradation of PAHs can be both negative and positive depending on type and concentration of both HMs and PAHs. In both rhizospheric and non-rhizospheric soils, the pyrene degradation was in line with the changes of bacterial structure, increasing number of pyrene degraders, and the prevalence of dioxygenase genes (nahAc and pdo1). This work represents the first report that Pb can affect the dissipation of pyrene and functional genes were detected in both rhizospheric and non-rhizospheric soils amended with pyrene and Pb/pyrene.
Conclusions
Pyrene removal efficiency for rhizospheric soils was higher than for non-rhizospheric soils and pyrene dissipation was accelerated in the presence of Pb in both rhizospheric and non-rhizospheric soils. The bacterial community structure was changed and the addition of pyrene indicated dramatic effects on the number of pyrene degraders. The catabolic genes, including nahAc and pdo1, which are responsible for HMW-PAH degradation, were confirmed in both rhizosphere and non-rhizosphere soils amended with pyrene or Pb/pyrene.
Recommendations and perspectives
Our findings suggest that the interaction between bacterial community and plant roots could influence the PAH degradation both in the presence and absence of HMs in the contaminated soils.



Similar content being viewed by others
References
Arthur EL, Rice PJ, Rice PJ, Anderson TA, Baladi SM, Henderson KLD, Coats JR (2005) Phytoremediation—an overview. Crit Rev Plant Sci 24:109–122
Baldwin BR, Nakatsu CH, Nies L (2003) Detection and enumeration of aromatic oxygenase genes by multiplex and real-time PCR. Appl Environ Microb 69:3350–3358
Brinch UC, Ekelund F, Jacobsen CS (2002) Method for spiking soil samples with organic compounds. Appl Environ Microb 68:1808–1816
Chadhain SMN, Sean Norman R, Pesce KV, Kukor JJ, Zylstra GJ (2006) Microbial dioxygenase gene population shifts during polycyclic aromatic hydrocarbon biodegradation. Appl Environ Microb 72:4078–4087
Hamamura N, Olson SH, Ward DM, Inskeep WP (2006) Microbial population dynamics associated with crude-oil biodegradation in diverse soils. Appl Environ Microb 72:6316–6324
Hamann C, Hegemann J, Hildebrandt A (1999) Detection of polycyclic aromatic hydrocarbon degrading genes in different soil bacteria by polymerase chain reaction and DNA hybridization. FEMS Microbiol Lett 173:255–263
Hatzinger PB, Alexander P (1995) Effect of ageing of chemicals in soil on their biodegradability and extractability. Environ Sci Technol 29:537–545
Hutchinson SL, Schwab AP, Banks HK (2003) Biodegradation of ptroleum hydrocarbons in the rhizosphere. In: McCutcheon SC, Schnoor JL (eds) Phytoremediation: transformation and control of contaminants. Wiley-Interscience, Inc., Hoboken, NJ, pp 355–385
Johnsen AR, Lipthay JR, Sørensen SJ, Ekelund F, Christensen P, Andersen O, Karlson U, Jacobsen CS (2006) Microbial degradation of street dust polycyclic aromatic hydrocarbons in microcosms simulating diffuse pollution of urban soil. Environ Microbiol 8:535–545
Johnsen AR, Schmidt S, Hybholt TK, Henriksen S, Jacobsen CS, Andersen O (2007) Strong impact on the polycyclic aromatic hydrocarbon (PAH)-degrading community of a PAH-polluted soil but marginal effect on PAH degradation when priming with bioremediated soil dominated by mycobacteria. Appl Environ Microbiol 73:1474–1480
Joner EJ, Leyval C (2003) Rhizosphere gradients of polycyclic aromatic hydrocarbon (PAH) dissipation in two industrial soils and the impact of arbuscular Mycorrhiza. Environ Sci Technol 37:2371–2375
Jouanneau Y, Willison JC, Mayer C, Krivobok S, Chevron N, Besombes JL, Blake G (2005) Stimulation of pyrene mineralization in freshwater sediments by bacterial and plant bioaugmentation. Environ Sci Technol 39:5729–5735
Kamath R, Schnoor JL, Alvarez PJJ (2004) Effect of root-derived substrates on the expression of nah-lux genes in pseudomonas fluorescens HK44: implications for PAH biodegradation in the rhizosphere. Environ Sci Technol 38:1740–1745
Kaplan CW, Kitts CL (2004) Bacterial succession in a petroleum land treatment unit. Appl Environ Microbiol 70:1777–1786
Khan AA, Wang RF, Cao WW, Doerge DR, Wennerstrom D, Cerniglia CE (2001) Molecular cloning, nucleotide sequence, and expression of genes encoding a polycyclic aromatic ring dioxygenase from Mycobacterium sp. strain PYR-1. Appl Environ Microbiol 67:3577–3585
Khan S, Cao Q, Chen B, Zhu YG (2006) Humic acids increase the phytoavailability of Cd and Pb to wheat plants cultivated in freshly spiked, contaminated soil. J Soils Sediments 6:236–242
Khan S, Cao Q, Hesham AB, Xia Y, He J (2007) Soil enzymatic activities and microbial community structure with different application rates of Cd and Pb. J Environ Sci 19:834–840
Khan S, Cao Q, Lin AJ, Zhu YG (2008a) Concentrations and bioaccessibility of polycyclic aromatic hydrocarbons in wastewater-irrigated soil using in vitro gastro-intestinal test. Environ Sci Pollut Res 15:344–353
Khan S, Aijun L, Zhang S, Hu Q, Zhu YG (2008b) Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in contaminated soils from long-term wastewater irrigation. J Hazard Mat 152:506–515
Kim SJ, Kweon O, Freeman JP, Jones RC, Adjei MD, Jhoo JW, Edmondson RD, Cerniglia CE (2006) Molecular cloning and expression of genes encoding a novel dioxygenase involved in low- and high-molecular-weight polycyclic aromatic hydrocarbon degradation in Mycobacterium vanbaalenii PYR-1. Appl Environ Microbiol 72:1045–1054
Krivobok S, Kuony S, Meyer C, Louwagie M, Willison JC, Jouanneau Y (2003) Identification of pyrene-induced proteins in Mycobacterium sp strain 6PY1: evidence for two ring-hydroxylating dioxygenases. J Bacteriol 185:3828–3841
Laurie AD, Lloyd-Jones G (1999) The phn genes of Burkholderia sp. strain RP007 constitute a divergent gene cluster for polycyclic aromatic hydrocarbon catabolism. J Bacteriol 181:531–540
Laurie AD, Lloyd-Jones G (2000) Quantification of phnAc and nahAc in contaminated New Zealand soils by competitive PCR. Appl Environ Microbiol 66:1814–1817
Leys NMEJ, Bastiaens L, Verstraere W, Springael D (2005) Influence of the carbon/nitrogen/phosphorus ratio on polycyclic aromatic hydrocarbons degradation by Mycobacterium and Sphingomonas in soil. Appl Environ Microbiol 66:726–736
Lloyd-Jones G, Laurie AD, Hunter DWF, Fraser R (1999) Analysis of catabolic genes for naphthalene and phenanthrene degradation in contaminated New Zealand soils. FEMS Microbiol Ecol 29:69–79
Luca C, Daniele A, Marisa M, Comi G (2002) An application of DGGE analysis to profile the yeast population in raw milk. Int Dairy J 12:407–411
Macleod CJA, Semple KT (2006) The influence of single and multiple applications of pyrene on the evolution of pyrene catabolism in soil. Environ Pollut 139:455–460
Moreau CJ, Klerks PL, Haas CN (1999) Interaction between phenanthrene and zinc in their toxicity to the sheepshead minnow (Cyprinodon variegatus). Arch Environ Contam Toxicol 37:251–257
Mueller KE, Shann JR (2006) PAH dissipation in spiked soil: impacts of bioavailability, microbial activity, and trees. Chemosphere 64:10061014
Muyzer G, de EC Waal, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Rentz JA, Alvarez P, Schnoor JL (2004) Repression of Pseudomonas putida phenanthrene-degrading activity by plant root extracts and exudates. Environ Microbiol 6:574–583
Shen G, Lu Y, Zhou Q, Hong J (2005) Interaction of polycyclic aromatic hydrocarbons and heavy metals on soil enzyme. Chemosphere 61:1175–1182
Shen G, Lu Y, Hong J (2006) Combined effect of heavy metals and polycyclic aromatic hydrocarbons on urease activity in soil. Ecotox Environ Safety 63:474–480
Stapleton RD, Savage DC, Sayler GS, Stacey G (1998) Biodegradation of aromatic hydrocarbons in an extremely acidic environment. Appl Environ Microbiol 64:4180–4184
Stuart-Keil KG, Hohnstock AM, Drees KP, Herrick JB, Madsen EL (1998) Plasmids responsible for horizontal transfer of naphthalene catabolism genes between bacteria at a coal tar-contaminated site are homologous to pDTG1 from Pseudomonas putida NCIB 9816–4. Appl Environ Microbiol 64:3633–3640
Su YH, Zhu YG (2007) Transport mechanisms for the uptake of organic compounds by rice (Oryza sativa) roots. Environ Pollut 148:94–100
Tang L, Tang XY, Zhu YG, Zheng MH, Miao QL (2005) Contamination of polycyclic aromatic hydrocarbons (PAHs) in urban soils in Beijing, China. Environ Int 31:822–828
US-EPA (2000) Introduction to phytoremediation. US Environmental Protection Agency, Publication No. 600/R-99/107
Widada J, Nojiri H, Kasuga K, Yoshida T, Habe H, Omori T (2002) Molecular detection and diversity of polycyclic aromatic hydrocarbon-degrading bacteria isolated from geographically diverse sites. Appl Environ Microbiol 58:202–209
Wu Y, Luo Y, Zou D, Ni J, Liu W, Teng Y, Li Z (2008) Bioremediation of polycyclic aromatic hydrocarbons contaminated soil with Monilinia sp.: degradation and microbial community analysis. Biodegradation 19:247–257
Xu SY, Chen YX, Wu WX, Wang KX, Lin Q, Liang XQ (2006) Enhanced dissipation of phenanthrene and pyrene in spiked soils by combined plants cultivation. Sci Total Environ 363:206–215
Yi H, Crowley DE (2007) Biostimulation of PAH degradation with plants containing high concentrations of linoleic acid. Environ Sci Technol 41:4382–4388
Yoon J, Cao X, Zhou Q, Ma LM (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Acknowledgments
This research was financially supported by Chinese Ministry of Science and Technology (2007CB407301 and 007CB407304), Chinese Academy of Sciences (KZCX1-YW-06-03). We thank Dr. Patrick O'Connor and Ms. Margaret Cargill, the University of Adelaide, Australia, for their critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, S., El-Latif Hesham, A., Qing, G. et al. Biodegradation of pyrene and catabolic genes in contaminated soils cultivated with Lolium multiflorum L. J Soils Sediments 9, 482–491 (2009). https://doi.org/10.1007/s11368-009-0061-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-009-0061-5