Abstract
Purpose
The nitrification inhibitor dicyandiamide (DCD) has been shown to be highly effective in reducing nitrate (NO3 −) leaching and nitrous oxide (N2O) emissions when used to treat grazed pasture soils. However, there have been few studies on the possible effects of long-term DCD use on other soil enzyme activities or the abundance of the general soil microbial communities. The objective of this study was to determine possible effects of long-term DCD use on key soil enzyme activities involved in the nitrogen (N) cycle and the abundance of bacteria and archaea in grazed pasture soils.
Materials and methods
Three field sites used for this study had been treated with DCD for 7 years in field plot experiments. The three pasture soils from three different regions across New Zealand were Pukemutu silt loam in Southland in the southern South Island, Horotiu silt loam in the Waikato in the central North Island and Templeton silt loam in Canterbury in the central South Island. Control and DCD-treated plots were sampled to analyse soil pH, microbial biomass C and N, protease and deaminase activity, and the abundance of bacteria and archaea.
Results and discussion
The three soils varied significantly in the microbial biomass C (858 to 542 μg C g−1 soil) and biomass N (63 to 28 μg N g−1), protease (361 to 694 μg tyrosine g−1 soil h−1) and deaminase (4.3 to 5.6 μg NH4 + g−1 soil h−1) activity, and bacteria (bacterial 16S rRNA gene copy number: 1.64 × 109 to 2.77 × 109 g−1 soil) and archaea (archaeal 16S rRNA gene copy number: 2.67 × 107 to 3.01 × 108 g−1 soil) abundance. However, 7 years of DCD use did not significantly affect these microbial population abundance and enzymatic activities. Soil pH values were also not significantly affected by the long-term DCD use.
Conclusions
These results support the hypothesis that DCD is a specific enzyme inhibitor for ammonia oxidation and does not affect other non-target microbial and enzyme activities. The DCD nitrification inhibitor technology, therefore, appears to be an effective mitigation technology for nitrate leaching and nitrous oxide emissions in grazed pasture soils with no adverse impacts on the abundance of bacteria and archaea and key enzyme activities.
Similar content being viewed by others
1 Introduction
In New Zealand, the major land use is intensively grazed pasture farming where the animals graze pastures year round. In such production systems, animal urine patches have been identified as the major source of nitrate (NO3 −) leaching and nitrous oxide (N2O) emissions (Jarvis et al. 1995; Di and Cameron 2000, 2002a; De Klein et al. 2003). The N loading rate under a dairy cow urine patch may range between about 700 and 1,200 kg N ha−1, which is far greater than the N requirements of pastures (Haynes and Williams 1993; Jarvis et al. 1995). Thus, the excess N in the soil can lead to NO3 −–N leaching and N2O emissions. These soil N losses represent both an inefficiency of N use in the agricultural system and also an environmental issue (Di and Cameron 2002a). Hence, major efforts have been devoted to developing mitigation strategies to reduce N losses from the animal urine patches in grazed pastures.
One highly effective mitigation technology for reducing NO3 − leaching and N2O emissions from grazed pasture soil is the use of nitrification inhibitors such as dicyandiamide (DCD) (Di and Cameron 2002b, 2003, 2004, 2007; Di et al. 2007; Monaghan et al. 2009; de Klein et al. 2011). DCD works by deactivating the ammonia monooxygenase enzyme responsible for the first step of the nitrification process, the conversion of ammonia to hydroxylamine (Amberger 1989; Di et al. 2009). It is a non-toxic, non-volatile and water-soluble compound and is degraded to CO2, NH4 + and H2O without leaving any long-term residues in the soil (Amberger 1989). More recent studies have shown that the use of DCD to treat grazed pasture soils did not adversely affect other soil agrobacteria and specific microbial communities such as methanotrophs (O’Callaghan et al. 2010; Di et al. 2011). However, possible long-term effects of repeated DCD use on other soil microbial enzyme activities and population abundance have not been widely studied. Such information is important for the long-term, sustainable use of DCD as an environmental mitigation tool.
The objective of this study was, therefore, to determine possible effects of long-term DCD use on key soil microbial enzyme activities involved in the nitrogen cycle, microbial biomass and general soil bacteria and archaea population abundance in three different grazed pasture soils which had been treated with DCD for 7 years.
2 Materials and methods
2.1 Soils
Three long-term field plot trials were established in 2004 to study the effect of DCD on nitrate leaching and/or pasture yield at three different locations across New Zealand: Southland (SL, southern South Island), Canterbury (CT, central South Island) and the Waikato (WK, central North Island) (see Table 1 for coordinates). The Southland site was located on a dairy farm at Tussock Creek about 20 km north of Invercargill (see Monaghan et al. 2009 for details). The SL soil is a poorly drained Pukemutu silt loam (NZ classification: Argillic-mottled Fragic Pallic soil; USDA: Aeric Fragiaquept) (Monaghan et al. 2009). The pasture was dominated by a mixture of perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.). Twelve hydrologically-isolated plots (12 × 15 m) were established. Mole (installed at 450 mm in depth) pipe (installed at 750 mm) drainage systems were installed in each plot to collect drainage water. Two treatments, control and DCD, each with six replicates, were allocated to the plots in a randomized block design. DCD was applied at 10 kg ha−1 (active ingredient) to the DCD plots two or three times per year between autumn and early spring. The plots were grazed as per the normal grazing cycle of the dairy farm. The plots also received basal and N fertilizers as per normal farm practice (Monaghan et al. 2009).
The Canterbury site (CT) was located on the Lincoln University demonstration dairy farm, about 20 km south of Christchurch in the central South Island (see Table 1). The CT soil is a free-draining Templeton silt loam (NZ classification: Immature Pallic soil; USDA: Udic Haplustepts). Sixteen plots (20 × 7 m) were established and were fenced off from grazing. Treatments included control and DCD which were randomly allocated to the plots. DCD was applied to the DCD treatments at 10 kg ha−1 (active ingredient) twice per year, once in late autumn and once in early spring. The pasture, which was predominantly perennial ryegrass (L. perenne L.) and white clover (T. repens L.), was cut at intervals, similar to the grazing rotation of the dairy farm, and was removed. The plots received normal fertilizer applications as per the farm operations. The plots also received the same amount of irrigation water as the rest of the farm during the summer, typically about 500 mm per year. Soil samples were taken from control plots where no animal urine was applied during the 7-year period.
The Waikato site was located on a dairy farm in the central North Island. The soil is a Horotiu silt loam (NZ classification: Typic Orthic Allophanic soil; USDA: Typic Udivitrand). This soil is a free-draining soil developed from volcanic parent material. The pasture was a mixture of perennial ryegrass (L. perenne L.) and white clover (T. repens L.). Twelve field plots, 24.5 × 29.5 m each, were established on the Horotiu soil. Three treatments were applied to the plots: a control, DCD applied twice per year (10–15 kg DCD ha−1, once in late autumn and once in mid-winter or early spring) and DCD applied three times per year (once in late autumn, once in mid-winter and once in spring). The plots were grazed as per the normal grazing cycle of the farm. The plots also received the same N and other fertilizers as the rest of the farm. However, soil samples for this study were only collected from the control treatment and the plots where DCD was applied twice per year.
2.2 Soil sampling
Soil samples (0–0.1 m in depth) were collected before DCD application in autumn (April) 2011 to determine the effect of DCD use since 2004. Twenty random samples were collected from each plot and bulked into a single sample. The samples were packed with ice blocks and kept in a cool room before being analysed.
2.3 Biochemical analysis
Soil pH was measured in de-ionised water (soil/water ratio, 1:2.5). Soil microbial biomasses C and N were determined by using the chloroform fumigation extraction method as described by Vance et al. (1987) and Brookes et al. (1985). Two 5-g samples of moist soil were taken from each replicate. Half of the samples were chloroform fumigated for 24 h and then extracted with 0.5 M K2SO4 by shaking at 120 rpm. The suspended samples were centrifuged and filtered. The other set of samples (controls) were unfumigated but were extracted in the same way. Total C and N in these filtrates were analysed using a total organic carbon analyser and a flow injection analyser, respectively. Microbial biomasses C and N were calculated as the difference between the amounts of C and N extracted from the fumigated and unfumigated samples.
Soil protease activity was assayed using the method of Ladd and Butler (1972), as modified by Burton and McGill (1992). Four grams of moist soil was weighed into a 50-mL centrifuge tube and 10 mL of sodium caseinate (10 mg mL−1 in 0.1 M tris(hydroxymethyl)aminomethane (TRIS) buffer (pH = 8.1) added. The mixture was shaken at 120 oscillations per minute on a reciprocating shaker bath at 50 °C. After 1 h, the reaction was stopped with 4 mL of 17.5 % trichloroacetic acid, and then, the tubes were centrifuged at 2,000×g for 20 min. Two millilitres of supernatant was removed and added to 3.0 mL of 1.4 M Na2CO3 and 1 mL of threefold diluted Folin & Ciocalteu’s phenol reagent (BDH). For soil controls, 0.1 M TRIS buffer was substituted for the caseinate-buffer solution. Reagent controls included all reagents but no soil. Absorbance at 700 nm was measured after 10 min using a solution spectrophotometer and then compared to tyrosine standards.
Soil deaminase activity was measured using the procedure as described by Alef and Kleiner (1987). Five grams of moist soil was incubated at 37 °C for 3 h after adding 2 mL of 11.5 mM arginine solution. A blank was similarly prepared but immediately frozen at −20 °C. After incubation, 18 mL of 2 M KCl solution was added to the soil, shaken for 30 min and filtered. Three millilitres KCl solution, 2 mL of 0.12 M sodium phenolate solution, 1 mL of 0.17 mM sodium nitroprusside and 1 mL sodium hypochlorite solution were added to 1 mL of the filtrate. After 30 min, the colour formed was measured spectrophotometrically against the blank at 630 nm.
2.4 Determination of bacterial and archaeal abundance
Bacterial and archaeal 16S rRNA gene abundance were quantified using real-time quantitative PCR (qPCR). DNA was extracted from 0.4 g soil (fresh weight) using MoBio PowersoilTM DNA isolation kits (MoBio Laboratories, GeneWorks Pty Ltd, South Australia, Australia) according to the manufacturer’s instructions. Four replicated DNA extractions were prepared for each treatment at each location. Concentration and quality of the extracted DNA were estimated using Qubit fluorometer (Invitrogen, Auckland, New Zealand) and NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies, Montchanin, USA). All DNA extractions were diluted 1:10 to reduce potential PCR inhibition. PCR primer pair 1369F and 1492R (Suzuki et al. 2000), and primer pair A364aF and A934bR (Burggraf et al. 1997; Großkopf et al. 1998) were used to amplify regions of the bacterial and archaeal 16S rRNA genes, respectively (Table 2). All reactions were set up using the CAS-1200 Robotic liquid handling system (Corbett Life Science, BioStrategy, Auckland, New Zealand), and real-time qPCR was performed with SYBR® Premix Ex TaqTM master mix (TaKaRa, Norrie Biotech, Auckland, New Zealand) on a Rotor-GeneTM 6000 real-time rotary analyser (Corbett Life Science, BioStrategy, Auckland, New Zealand). A typical 16-μL reaction consisted of 8.0 μL of SYBR®Premix Ex TaqTM, 0.5 μL each primer (10 μM) for bacteria or 0.2 μL each primer (10 μM) for archaea, 1.5 μL of template DNA and sterile water to the final volume. The PCR cycling conditions are detailed in Table 2.
Standards used in the qPCR analysis were prepared as follows. The archaeal and bacterial 16S rRNA gene fragments from the extracted DNA using Premix Ex TaqTM master mix (TaKaRa, Norrie Biotech, Auckland, New Zealand) with primers and conditions are listed in Table 2. The PCR products were then gel purified using AxyPrep DNA Gel Extraction Kit (Axygen, Total Laboratory Systems, Auckland, New Zealand), cloned into the pGEM-T Vector (Promega, In Vitro Technologies, Auckland, New Zealand) and transformed into JM109 High Efficiency Competent Cells (Promega, In Vitro Technologies, Auckland, New Zealand), according to the manufacturer’s recommendations. The transformed cells were then grown in 3 mL of Lurea Bertani medium overnight, and plasmids containing correct inserts were extracted and purified using PureLinkTM Quick Plasmid Miniprep Kit (Invitrogen, Auckland, New Zealand). The concentration and quality of the extracted plasmid DNA were estimated as described earlier. Standard curves used for each gene quantification were generated using a series of 1:10 dilutions of plasmid DNA, giving a concentration range from 102 to 108 copies per microlitre. Amplification efficiencies of 95–101 % (R 2 > 0.99) and 92–99 % (R 2 > 0.99) were observed for the bacterial and archaeal standards, respectively.
A typical qPCR run included soil DNA samples, corresponding standards and no-template control reactions. Data analysis was carried out using Rotor-GeneTM 6000 series software 1.7 (Corbett Life Science, BioStrategy, Auckland, New Zealand).
2.5 Data analysis
Mean values and standard errors of all data were calculated based on the replicates of each treatment. Analysis of variance was performed using Genstat Version 14 (Lawes Agricultural Trust) to test the effect of DCD use on the soil properties.
3 Results
3.1 Soil pH
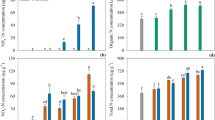
The pH in the three soils varied slightly from 5.9 in the SL soil to 6.3 in the CT soil (Fig. 1). However, there was no significant difference in soil pH between the control and DCD treatment in all the three soils.
3.2 Microbial biomasses C and N
Microbial biomass carbon varied from 858 μg C g−1 soil in the SL soil to 542 μg C g−1 soil in the CT soil (Fig. 2). The application of DCD did not significantly affect the soil microbial biomass carbon in the three soils. Soil microbial biomass N varied from 63 μg N g−1 soil in the SL soil to 28 μg N g−1 soil in the CT soil (Fig. 3). Similarly, the application of DCD over 7 years did not result in significant changes in microbial biomass N in the three soils.
3.3 Soil protease and deaminase activity
Soil protease activity varied from 361 μg tyrosine g−1 soil h−1 in the SL soil to 694 μg tyrosine g−1 soil h−1 in the CT soil (Fig. 4). The use of DCD did not significantly change the soil protease activity. The soil deaminase varied from 4.3 μg NH4 + g−1 soil h−1 in the WK soil to 5.6 μg NH4 + g−1 soil h−1 in the CT soil (Fig. 5). The DCD treatment did not result in a significant difference in deaminase activity in the three soils.
3.4 Abundance of bacteria and archaea
The bacteria DNA gene copy numbers varied from 1.64 × 109 g−1 soil in the WK control soil to 2.77 × 109 g−1 in the SL control soil (Fig. 6). After 7 years of DCD treatment, there was no significant difference in the bacteria gene copy numbers between the control and DCD treatment. The archaea gene copy numbers varied significantly between the three soils but were more than tenfold lower than the bacteria gene copy numbers (Fig. 7). Overall, the use of DCD did not significantly affect the archaea gene copy numbers. The archaea gene copy numbers in the DCD treatment were slightly higher than in the control treatment for the CT soil. Further research is required to determine the significance of this difference.
4 Discussion
The nitrification inhibitor DCD is increasingly used in New Zealand as a means of mitigating NO3 − leaching and N2O emissions in grazed pastures. One of the questions arising from this practice is whether the long-term use of DCD has an impact on soil microbial communities and key N cycling processes (e.g. N mineralization). Results from this study clearly show that after 7 years of DCD use on three different grazed dairy pasture soils across different parts of New Zealand, no significant effect was observed on soil microbial C and N levels, protease and deaminase activities, and the abundance of bacteria and archaea in the three soils. These results would support the hypothesis that DCD is a specific inhibitor for ammonia oxidizers, which carry the ammonia monooxygenase gene, and does not affect the abundance of bacteria and archaea and the key enzyme activities which are important for the N cycle.
Soil microbial biomass is a relatively small but an important component of the soil organic matter and is often used as a sensitive indicator for soil quality change. The lack of a significant effect following 7 years of DCD use would suggest that DCD appears to be benign to the bulk of the soil microbial communities while specifically inhibiting the growth of ammonia oxidizers (Di et al. 2009). This conclusion is further supported by the lack of a significant impact on bacteria and archaea in the three soils. Similarly, O’Callaghan et al. (2010) also found that DCD was relatively benign and did not affect the soil microbial communities. Di et al. (2011) studied the effect of DCD on a specific group of the soil microbial community and methanotrophs, and also found no significant effect. Other studies (e.g. Di and Cameron 2004; Zaman et al. 2009) also found no effect of DCD on soil microbial biomass. The significance of the slightly higher archaea gene copy numbers in the DCD treatment in the CT soil is unclear and will require further investigation.
The two soil enzymes, protease and deaminase, chosen for this study are important for N cycling in the soil, responsible for the sequential mineralization of soil organic N. Soil enzyme activities and microbial biomass are closely related and are sometimes also used as indices of soil quality. The lack of a significant effect after 7 years of DCD use on the activity of the two enzymes would suggest that DCD can specifically inhibit one step of the nitrogen cycle, namely the oxidation of ammonia, whilst not affecting other key soil N cycling processes.
5 Conclusions
These results and those reported earlier would support the conclusion that DCD is a relatively benign nitrification inhibitor which specifically targets the inhibition of the ammonia oxidation process and which has little or no long-term effect on the abundance of bacteria and archaea and key soil enzyme activities that are responsible for N cycling in the soil.
References
Alef K, Kleiner D (1987) Applicability of arginine ammonification as indicator of microbial activity in different soils. Biol Fert Soils 5:148–151
Amberger A (1989) Research on dicyandiamide as a nitrification inhibitor and future outlook. Commun Soil Sci Plan 20:1933–1955
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release and extractability of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Burggraf S, Huber H, Stetter KO (1997) Reclassification of the crenarchaeal orders and families in accordance with 16S rRNA sequence data. Int J Syst Bacteriol 47:657–660
Burton DL, McGill WB (1992) Spatial and temporal fluctuation in biomass, nitrogen mineralizing reactions and mineral nitrogen in a soil cropped to barley. Can J Soil Sci 72:31–42
De Klein CAM, Barton L, Sherlock RR, Li Z, Littlejohn RP (2003) Estimating a nitrous oxide emission factor for animal urine from some New Zealand pastoral soils. Aust J Soil 41:381–399
De Klein CAM, Cameron KC, Di HJ, Rys G, Monaghan R, Sherlock RR (2011) The effect of long-term use of the nitrification inhibitor DCD on reducing N2O emissions from cow urine. Anim Feed Sci Techn 166–167:480–491
Di HJ, Cameron KC (2000) Calculating nitrate leaching losses and critical nitrogen application rates in dairy pasture systems using a semi-empirical model. New Zeal J Agr Res 43:139–147
Di HJ, Cameron KC (2002a) Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr Cycl Agroecosys 64:237–256
Di HJ, Cameron KC (2002b) The use of a nitrification inhibitor, dicyandiamide (DCD), to decrease nitrate leaching and nitrous oxide emissions in simulated grazed and irrigated grassland. Soil Use Manage 18:395–403
Di HJ, Cameron KC (2003) Mitigation of nitrous oxide emissions in spry-irrigated grazed by treating the soil with dicyandiamide, a nitrification inhibitor. Soil Use Manage 19:84–290
Di HJ, Cameron KC (2004) Effects of temperature and application rate of a nitrification inhibitor, dicyandiamide (DCD), on nitrification rate and microbial biomass in a grazed pasture soil. Aust J Soil Res 42:927–932
Di HJ, Cameron KC (2007) Nitrate leaching losses and pasture yields as affected by different rates of animal urine nitrogen returns and application of a nitrification inhibitor-a lysimeter study. Nutr Cycl Agroecosys 79:281–290
Di HJ, Cameron KC, Sherlock RR (2007) Comparison of the effectiveness of a nitrification inhibitor, dicyandiamide, in reducing nitrous oxide emissions in four different soils under different climatic and management conditions. Soil Use Manage 23:1–9
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen rich grassland soils. Nat Geosci 2:621–624
Di HJ, Cameron KC, Shen JP, Winefield CS, O'Callaghan M, Bowatte S, He JZ (2011) Methanotroph abundance not affected by applications of animal urine and a nitrification inhibitor, dicyandiamide, in six grazed grassland soils. J Soils Sediments 11:1032–1039
Großkopf R, Janssen PH, Liesack W (1998) Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microb 64:960–969
Haynes RJ, Williams PH (1993) Nutrient cycling and soil fertility in the grazed pasture ecosystem. Adv Agron 49:119–199
Jarvis SC, Scholefield D, Pain B (1995) Nitrogen cycling in grazing systems. In: Bacon PE (ed) Nitrogen fertilization in the environment. Marcel Dekker Inc., New York, pp 381–419
Ladd JM, Butler JH (1972) Short-term assays of soil proteolytic enzyme activity using proteins and dipeptide derivates as substrates. Soil Biol Biochem 4:19–30
Monaghan RM, Smith LC, Ledgard SF (2009) The effectiveness of a granular formulation of dicyandiamide (DCD) in limiting nitrate leaching from a grazed dairy pasture. New Zeal J Agr Res 52:145–159
O’Callaghan M, Gerard EM, Carter PE, Lardner R (2010) Effect of the nitrification inhibitor dicyandiamide (DCD) on microbial communities in a pasture soil amended with bovine urine. Soil Biol Biochem 42:1425–1436
Suzuki MT, Taylor LT, DeLong EF (2000) Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5’-nuclease assays. Appl Environ Microbiol 66:4605–4614
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Zaman M, Saggar S, Blennerhassett JD, Singh J (2009) Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biol Biochem 41:1271–1280
Acknowledgments
We thank the New Zealand Ministry of Business, Innovation and Employment, and the New Zealand Agricultural Greenhouse Research Centre for funding this programme, and the China Scholarships Council for funding Yan-Jie Guo to study in New Zealand; and Trevor Hendry, Steve Moore, Neil Smith, Nigel Beale, Carole Barlow, Jie Lei and Roger Atkinson of Lincoln University, and Chris Roach and Rodger Jensen of DairyNZ in Hamilton for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yanfen Wang
Rights and permissions
About this article
Cite this article
Guo, Y.J., Di, H.J., Cameron, K.C. et al. Effect of 7-year application of a nitrification inhibitor, dicyandiamide (DCD), on soil microbial biomass, protease and deaminase activities, and the abundance of bacteria and archaea in pasture soils. J Soils Sediments 13, 753–759 (2013). https://doi.org/10.1007/s11368-012-0646-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-012-0646-2